Your enquiry has been submitted
Your enquiry has been submitted
Pseudomonas Agar Base
Intended use
For selective isolation of Pseudomonas species. The composition and performance criteria of this medium are as per the specifications laid down in ISO 16266-1:2006
Composition**
ISO specification - Pseudomonas agar base/CN-agar
| Ingredients | g/L |
|---|---|
| Gelatin peptone | 16.000 |
| Casein hydrolysate | 10.000 |
| Potassium sulphate, anhydrous (K2SO4) | 10.000 |
| Magnesium chloride, anhydrous (MgCl2) | 1.400 |
| Agar | 11.00-18.00 |
| Final pH (of solidified, complete medium) | 7.1±0.2 |
Pseudomonas Agar Base M085
| Ingredients | g/L |
|---|---|
| Gelatin peptone | 16.000 |
| Tryptone | 10.000 |
| Potassium sulphate | 10.000 |
| Magnesium chloride | 1.400 |
| Agar | 11.000 |
| Final pH (at 25°C) | 7.1±0.2 |
Supplement to be added after autoclaving
| g/L | Cetrinix Supplement - FD029 | mg / vial | |
|---|---|---|---|
| Hexadecyitrimethyl ammonium bromide(Cetrimide) | 0.200 | Cetrimide | 100mg |
| Nalidixic acid | 0.150 | Nalidixic acid | 7.500mg |
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 24.2 grams in 500 ml purified/distilled water containing 5 ml glycerol. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C and aseptically add 1 vial of sterile rehydrated contents of Cetrinix Supplement (FD029). Mix well and pour into sterile Petri plates.
Note : Do not keep the molten agar for longer than 4 hours.
Principle And Interpretation
Pseudomonas Agar Base is as per the specification laid down in ISO 16266 for testing water quality by MPN method (1). It is also a modification of Kings A medium (2) which contains magnesium chloride and potassium sulphate to enhance pigment production. CetriNix supplement is specified for the selective isolation of Pseudomonas aeruginosa from water samples, it suppresses the growth of Klebsiella, Proteus and Providencia species. Lowbury and Collins (3) studied cetrimide as a selective agent.
Tryptone and gelatin peptone supplies nitrogenous and carbonaceous compounds, long chain amino acids, and other essential growth nutrients.
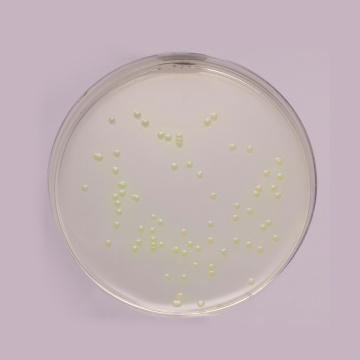
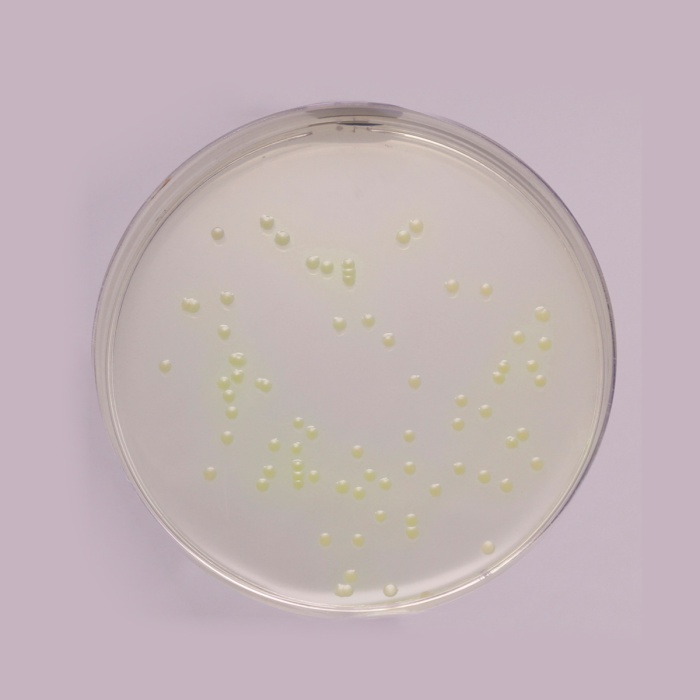
Examine inoculated plates after incubation at UV light 360 ± 20 nm. The presence of blue-green colonies with fluorescence may be considered as presumptive evidence of Pseudomonas aeruginosa.
Type of specimen
Clinical samples - pus, urine, Water samples.
Specimen Collection and Handling:
ISO 16266-1:2006
Preparation of test sample: Prepare tenfold dilutions of water samples (1,4). After use, contaminated materials must be sterilized by autoclaving before discarding.
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (5,6). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
In Vitro diagnostic Use. For professional use only. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Individual organisms differ in their growth requirement and may show variable growth patterns on the medium.
- Each lot of the medium has been tested for the organisms specified on the COA. It is recommended to users to validate the medium for any specific microorganism other than mentioned in the COA based on the user's unique requirement.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance
Cream to yellow homogeneous free flowing powder
Gelling
Firm, comparable with 1.1% Agar gel.
Colour and Clarity of prepared medium
Yellow coloured clear to slightly opalescent gel forms in Petri plates
Reaction
Reaction of 4.84% w/v aqueous solution containing 1% v/v glycerol at 25°C. pH: 7.1±0.2
pH
6.90-7.30
Cultural Response
Productivity: Cultural response was observed after an incubation at 36 ± 2°C for 44 ± 4 hours, with added sterile Cetrinix Supplement (FD029). Recovery rate is considered as 100% for bacteria growth on Reference medium - Soyabean Casein Digest Agar (Tryptone Soya Agar).
Selectivity: Cultural response was observed after an incubation at 36 ± 2°C for 44 ± 4 hours, with added sterile Cetrinix Supplement (FD029).
| Organisms | Inoculum (CFU) | Growth | Recovery# | Fluorescence under UV 360±20 nm |
|---|---|---|---|---|
| Productivity | ||||
| Pseudomonas aeruginosa ATCC 27853 (00025*) | 50-100 | good-luxuriant | >=50% | blue-green with Fluorescence |
| ^Pseudomonas paraeruginosa ATCC 9027 (00026*) | 50-100 | good-luxuriant | >=50% | blue-green with Fluorescence |
| Pseudomonas aeruginosa ATCC 10145 (00024*) | 50-100 | good-luxuriant | >=50% | blue-green with Fluorescence |
| Selectivity | ||||
| Enterococcus faecalis ATCC 29212 (00087*) | >=104 | inhibited | 0% | |
| Enterococcus faecalis ATCC 19433 (00009*) | >=104 | inhibited | 0% | |
| Escherichia coli ATCC 25922 (00013*) | >=104 | inhibited | 0% | |
| Escherichia coli ATCC 8739 (00012*) | >=104 | inhibited | 0% | |
Key: (*) Corresponding WDCM numbers, ^ Formerly known as Pseudomonas aeruginosa # - Recovery obtained for productivity is >=70% when compared to a previously validated batch of Pseudomonas Agar Base (CN Agar) is used.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (5,6).
References
- Water quality - Detection and enumeration of Pseudomonas aeruginosa-- Method by membrane filtration; ISO 16266-1:2006
- King E.O., Ward M.K. and Raney D.E., 1954, J.Lab and Clin. Med., 44:301.
- Lowbury E.J. and Collins A.G., 1955, Clin. Path., 8:47.
- Lipps WC, Braun-Howland EB, Baxter TE,eds. Standard methods for the Examination of Water and Wastewater, 24th ed. Washington DC:APHA Press; 2023.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | Pseudomonas Agar Base |
|---|---|
| SKU | M085 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. King E.O., Ward M.K. and Raney D.E., 1954, J.Lab and Clin. Med., 44:301. 2.Goto S. and Entomoto S., 1970, Jap. J. Microbiol., 14:65. 3.Lowbury E.J. and Collins A.G., 1955, Clin. Path., 8:47. 4.Mead G.C. and Adams B.W., 1977, Br. Poult. Sci., 18:661. 5.Downes F. P. and Ito K., (Ed.), 2001, Compendium of Methods for the Microbiological Examination of Foods, 4th Ed.,American Public Health Association, Washington, D.C. 6.Greenberg A. E., Clesceri L. S. and Eaton A. D., (Eds.), 2005, Standard Methods for the Examination of Water andWastewater, 21st ed., APHA, Washington, D.C. 7.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition. 8.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |