Your enquiry has been submitted
Your enquiry has been submitted
Differential Reinforced Clostridial Agar
Intended Use
Recommended for the enumeration and cultivation of Clostridia from water and clinical samples.
Composition**
| Ingredients | g / L |
|---|---|
| Tryptone | 5.000 |
| Peptone | 5.000 |
| HM peptone B # | 8.000 |
| Yeast extract | 1.000 |
| Starch | 1.000 |
| Sodium acetate | 5.000 |
| Dextrose (Glucose) | 1.000 |
| L-Cysteine hydrochloride | 0.500 |
| Sodium bisulphite | 0.500 |
| Ferric ammonium citrate | 0.500 |
| Resazurin | 0.002 |
| Agar | 15.000 |
Final pH ( at 25°C) 7.1±0.2
**Formula adjusted, standardized to suit performance parameters
# Equivalent to Beef extract
Directions
Suspend 42.5 grams in 1000 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50 °C. Mix well and pour into sterile Petri plates.
Principle And Interpretation
Attenborough and Scarr (1) employed Differential Reinforced Clostridial Agar in conjunction with membrane filter for the count of Clostridium thermosaccharolyticum in sugar. This medium is also frequently employed for the investigation of intestinal flora, with added blood. It is also used for the total and Lactobacillus count of human and animal faeces and for determination of Bacteroides.
This medium has ingredients like tryptone, peptone and yeast extract, HM peptone B, which provide nitrogen source, essential nutrients and growth factors to the organisms. Glucose serves as carbon and energy source. Sodium bisulphite and ferric ammonium citrate forms the indicator system for sulphite reduction, which results in black colour colonies. Resazurin is a redox indicator which helps in detection of anaerobisis, in the medium.
Grind the material to be examined in a stomacher and prepare serial 10 fold dilutions in ¼ strength Ringers Solution (M525) or 0.1% Peptone Water (M028). Transfer 1 ml or 0.1 ml of the appropriate dilution (depending upon amount of the initial sample) to the bottom of a molten (45-50°C) Differential Reinforced Clostridial Agar tubes. Prepare duplicate tubes using the same procedure. Tighten the caps of the tubes. Heat one of the duplicate tubes (dilution tubes) to 80 ± 1°C for 10 minutes to kill vegetative cells. Incubate both heat shocked and non-heat shocked tubes at 35 ± 1°C for 5 days. Observe the blackening of tubes for sulphite reduction. Non-heat shocked tubes showing blackening must be subcultured to Differential Reinforced Clostridial Agar for confirmation. Blackening of the medium is presumptive evidence for the presence of sulphite reducing clostridia. Heat shocked tubes showing blackening are confirmed for clostridia. Alternatively, samples may be inoculated onto the surface of agar plates using streak plate, spread plate or pour plate technique. Medium in agar deeps may be inoculated using stab technique. Differential Reinforced Clostridial Agar may be used to overlay the membrane filter in the filtration technique. Incubate plates and tubes at 35± 2°C for 24-48 hours under anaerobic conditions.
Type of specimen
Water samples; Clinical sample- faeces;
Specimen Collection and Handling
For water samples follow appropriate techniques for handling specimens as per established guidelines (2).
For clinical samples, follow appropriate techniques for sample collection, processing as per guidelines and local standards (3,4).
After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
In Vitro diagnostic Use. For professional use only. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/ face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Further biochemical and serological testing is required for complete identification.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Cream to yellow homogeneous free flowing powder
Gelling: Firm, comparable with 1.5% Agar gel
Colour and Clarity of prepared medium: Light pink coloured, clear to slightly opalescent gel forms in Petri plates
Reaction: Reaction of 4.25% w/v aqueous solution at 25°C. pH : 7.1±0.2
pH: 6.90-7.30
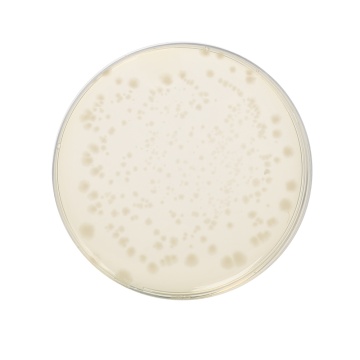
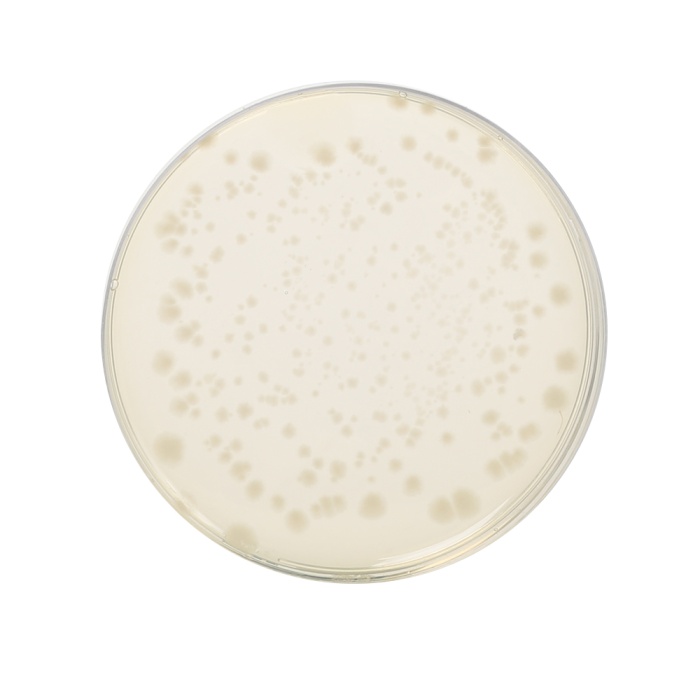
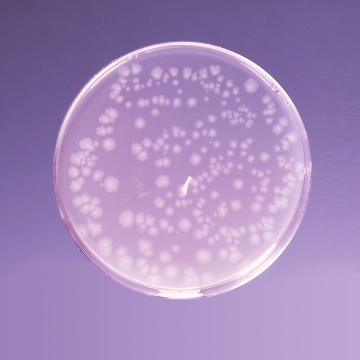
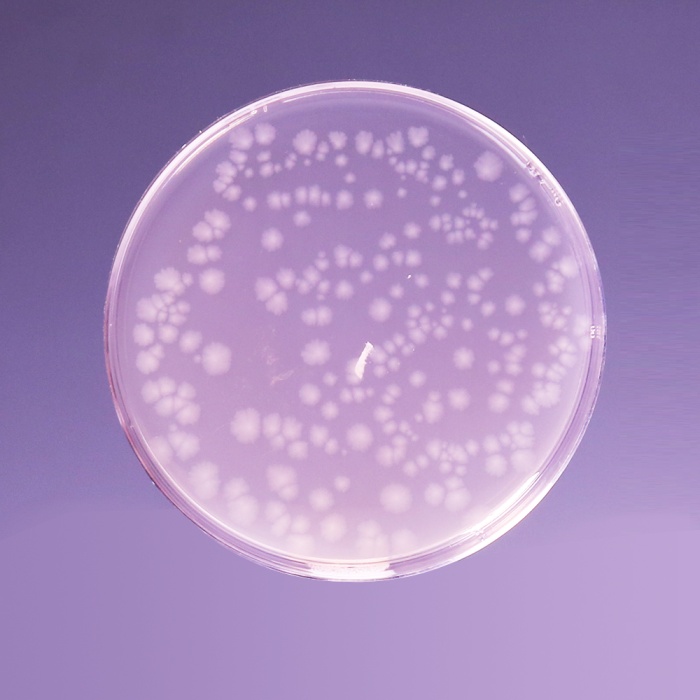
Cultural Response: Cultural characteristics observed in an anaerobic atmosphere, after an incubation at 30-35°C for 1 week.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| Clostridium perfringens ATCC 13124 (00007*) | 50-100 | good-luxuriant | >=50% | black |
| Clostridium sporogenes ATCC 11437 | 50-100 | good-luxuriant | >=50% | black |
Key : (*) Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (3,4).
Reference
- Attenborough Sheila J. and Scarr M. Pamela, 1957, J. Appl. Bacteriol., 20:460-466
- Lipps WC, Braun-Howland EB, Baxter TE,eds. Standard methods for the Examination of Water and Wastewater, 24th ed. Washington DC:APHA Press; 2023.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W.(2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | Differential Reinforced Clostridial Agar |
|---|---|
| SKU | M1603 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Attenborough Sheila J. and Scarr M. Pamela, 1957, J. Appl. Bacteriol., 20:460-466 |