Your enquiry has been submitted
Your enquiry has been submitted
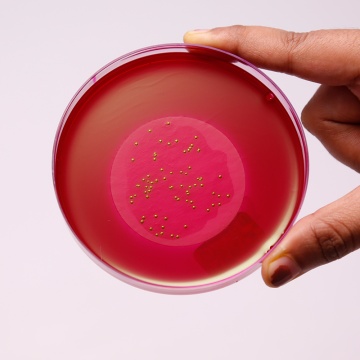
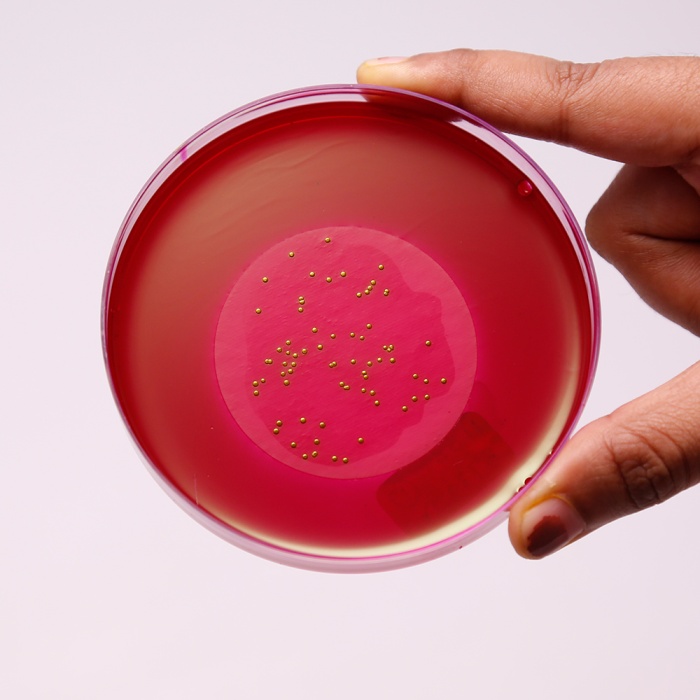
M-Endo Agar LES
Intended Use
Recommended for enumeration of coliforms in water using a two step membrane filtration technique.
Composition
| Ingredients | Gms / Litre |
|---|---|
| Tryptone | 3.700 |
| Peptone | 3.700 |
| Tryptose | 7.500 |
| Yeast extract | 1.200 |
| Lactose | 9.400 |
| Dipotassium hydrogen phosphate | 3.300 |
| Potassium dihydrogen phosphate | 1.000 |
| Sodium chloride | 3.700 |
| Sodium deoxycholate | 0.100 |
| Sodium lauryl sulphate (SLS) | 0.050 |
| Sodium sulphite | 1.600 |
| Basic fuchsin | 0.800 |
| Agar | 15.000 |
Final pH (at 25°C): 7.2±0.2
Formula adjusted, standardized to suit performance parameters
Directions
Suspend 51.05 grams in 980 ml purified/distilled water. Heat to boiling to dissolve the medium completely. DO NOT AUTOCLAVE. Cool to 45-50°C and aseptically add 20 ml of 95% ethanol. Mix and dispense 4 ml amounts into 60 mm Petri plates. In large plates, use sufficient medium to give 1.5 mm depth. DO NOT EXPOSE PLATES TO DIRECT SUNLIGHT.
Principle And Interpretation
It is possible to remove bacteria from fluids by passing them through filters with such small pore size that bacteria are arrested. This filtration technique enables fairly large volumes of water to pass rapidly under pressure, but prevents the passage of any bacteria present. These nutrients are retained on the surface of the membrane which is then brought into contact with suitable liquid nutrients. These diffuse upwards through the pores thereby inducing the organisms to grow as surface colonies which can be counted (1).
Endo Medium was first developed by Endo to differentiate between lactose-fermenters and non-fermenters (2). This medium employed sodium sulphite and basic fuchsin instead of bile salts to achieve inhibition of gram-positive bacteria (2). M-Endo Agar, LES is a modification of the original medium and is formulated as per McCarthy et al of Lawrence Experimental Station (LES) (7) for testing coliforms in water using a two-step membrane filter procedure, wherein Lauryl Sulphate Broth (M080) is used as the primary enrichment medium. This medium is recommended by APHA for testing coliforms in drinking and in bottled water (1, 5). Presumptive coliform bacteria will form red colonies with metallic sheen after an incubation at 35-37°C for 24 hours.
Tryptone, Tryptose, Peptone and yeast extract provide essential nutrients especially nitrogenous for the coliforms. Lactose is the fermentable carbohydrate. Sodium sulphite, sodium deoxycholate and basic fuchsin inhibit the growth of gram-positive organisms. Phosphates buffer the medium. Coliforms ferment lactose and the resulting acetaldehyde reacts with sodium sulphite and basic fuchsin to form red colonies and similar colouration of the medium. Lactose non-fermenters form colourless colonies.
In the first step of enrichment, cotton absorbent pad is impregnated with Lauryl Sulphate Broth (M080). Membrane filter through which water sample is passed is aseptically placed on it and incubated without inverting for 2 hours at 35°C in a humid atmosphere. After incubation, the membrane filter is aseptically transferred to the M-Endo Agar LES plate and incubated at 35°C for 24 hours. Alternatively membrane filter pad can be placed inside the lid of Petri plate of M-Endo Agar LES and then impregnated with 2 ml Lauryl Sulphate Broth (M080) and incubated for 1 - 12 hours at 35°C. In the second step, the prepared membrane filter is kept directly on the agar surface and incubated as described above. Presumptive coliforms produce golden green colonies with metallic sheen within 24 hours of incubation.
Coliform density calculation : Note the coliform density in terms of total coliforms/100 ml. Extrapolate the count using membrane filters with 20-80 coliform colonies but not more than 200 of all types per membrane.
The formula for calculating the count is as follows:
Total coliform colonies/100 ml = coliform colonies /ml of sample filtered x 100
Type of specimen
Water samples
Specimen Collection and Handling
For water samples, follow appropriate techniques for sample collection, processing as per guidelines and local standards (1).
After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets
Limitations
- Due to varying nutritional requirements, some strains may be encountered that grow poorly.
- If the inoculum is too heavy, the sheen may be suppressed.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Light pink to purple homogeneous free flowing powder
Gelling: Firm, comparable with 1.5% Agar gel
Colour and Clarity of prepared medium: Red coloured slightly opalescent gel forms in Petri plates
Reaction: Reaction of 5.1% w/v aqueous solution with 2% v/v alcohol at 25°C. pH : 7.2±0.2
pH: 7.00-7.40
Cultural Response
Cultural characteristics observed after an incubation at 35-37°C for 20 - 24 hours.
| Organism | Inoculum (CFU) | Growth | Colour of colony (on membrane filter) |
|---|---|---|---|
| Escherichia coli ATCC 25922 (00013*) | 50-100 | good-luxuriant | pink with metallic sheen |
| # Klebsiella aerogenes ATCC 13048 (00175*) | 50-100 | good-luxuriant | pink to red (may have sheen) |
| Salmonella Typhi ATCC 6539 | 50-100 | luxuriant | colourless to very light pink |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | >=104 | inhibited | |
| Klebsiella pneumoniae ATCC 13883 (00097*) | 50-100 | good-luxuriant | pink to red |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | colourless to very light pink |
Key: (*) Corresponding WDCM numbers.
(#) Formerly known as Enterobacter aerogenes
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (4,5).
Reference
- Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water and Wastewater, 23rd ed., APHA, Washington, D.C.
- Cruickshank R., Duguid J. P., Marmion B. P., Swain R. H. A., (Eds.), Medical Microbiology, 1975, 12th Ed. Vol. II, Churchill Livingstone
- Endo S., 1904, Zentralbl. Bakteriol., Abt. 1, Orig.35:109-110.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
- McCarthy J. A., Delaney J. E. and Grasso R., 1961, Water and Sewage Works, 108:238.
- Salfinger Y., and Tortorello M.L., 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
| Product Name | M-Endo Agar LES |
|---|---|
| SKU | M1106 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1.Cruickshank R., Duguid J. P., Marmion B. P., Swain R. H. A., (Eds.), Medical Microbiology, 1975, 12th Ed. Vol. II, ChurchillLivingstone2.Endo S., 1904, Zentralbl. Bakteriol., Abt. 1, Orig.35:109-110.3.McCarthy J. A., Delaney J. E. and Grasso R., 1961, Water and Sewage Works, 108:238.4.Eaton A. D., Clesceri L. S. and Greenberg A. W., (Eds.), 2005, Standard Methods for the Examination of Water andWastewater, 21st Ed., APHA, Washington, D.C.5.Downes F. P. and Ito K., (Eds.), 2001, Compendium of Methods for the Microbiological Examination of Foods, 4th Ed.,American Public Health Association, Washington, D.C. |