Your enquiry has been submitted
Your enquiry has been submitted
Casitose Soya Blood Agar Base
Intended use
Casitose Soya Blood Agar Base when supplemented with blood is recommended for cultivating fastidious microorganisms and study haemolytic reactions.
Composition**
| Ingredients | Gms / Litre |
|---|---|
| Tryptone, special | 15.000 |
| Soya peptone | 5.000 |
| Sodium chloride | 5.000 |
| Agar | 15.000 |
Final pH (at 25°C): 7.3±0.2
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 40 grams in 1000ml distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15lbs pressure (121°C) for 15 minutes. Cool to 45-50°C and aseptically add 7% sterile sheep blood. Mix well and pour into sterile Petri plates.
Principle And Interpretation
Casitose Soya Agar, Modified is a nutrient medium, which can be used as a base medium as well as an unsupplemented medium. Casitose Soya Agar, Modified is a modified version of Tryptone Soya Agar, which is supplemented with 5-10% sterile blood. This medium is used for cultivation of fastidious organisms and for determining haemolytic reactions. The medium can be used in differentiation of Streptococcus species. The medium is supplemented with growth factors to achieve a better growth of fastidious microorganisms. Blood is the most common additive for Tryptone Soya Agar and it can be added at different concentrations between 5 and 15%.
Tryptone, special and soya peptone in the medium provide organic nitrogen and amino acids. Sodium chloride maintains osmotic balance of the medium. Sheep blood stimulates excellent growth and aids in the formation of appropriate hemolytic reactions of fastidious organisms. The medium with 5% horse blood supplies both X and V factors that are growth requirements for certain organisms; e.g. Haemophilus influenzae. Haemolytic reactions displayed by defibrinated horse blood differ from those of sheep blood (1).
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Cream to yellow homogeneous free flowing powder
Gelling: Firm, comparable with 1.5% Agar gel
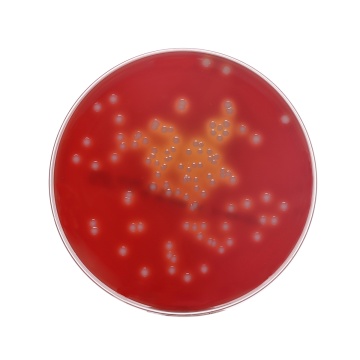
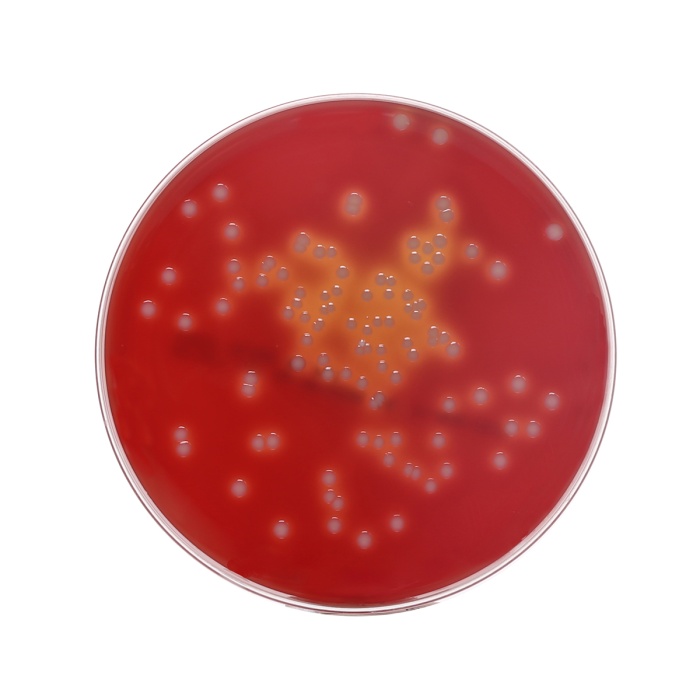
Colour and Clarity of prepared medium: Basal Medium : Light yellow coloured clear to slightly opalescent gel. After addition of 7%w/v sterile defibrinated blood : Cherry red coloured opaque gel forms in Petri plates
pH: 7.10-7.50
Cultural response: Cultural characteristics was observed after an incubation for Bacterial at 30-35°C 18-24 hours.
Cultural Response
| Organism | Inoculum (CFU) | Growth | Observed Lot value (CFU) | Recovery | Observed Lot value (CFU) w/blood | Recovery w/ blood |
|---|---|---|---|---|---|---|
| Streptococcus pyogenes ATCC 19615 | 50 -100 | luxuriant | 35 -100 | >=70 % | 35 -100 | 18 -24 hrs |
| Staphylococcus aureus ATCC 25923 (00034*) | 50 -100 | luxuriant | 35 -100 | >=70 % | 35 -100 | 18 -24 hrs |
| Staphylococcus aureus ATCC 6538 (00032*) | 50 -100 | luxuriant | 35 -100 | >=70 % | 35 -100 | 18 -24 hrs |
| Escherichia coli ATCC 25922 (00013*) | 50 -100 | luxuriant | 35 -100 | >=70 % | 35 -100 | 18 -24 hrs |
| Escherichia coli ATCC 8739 (00012*) | 50 -100 | luxuriant | 35 -100 | >=70 % | 35 -100 | 18 -24 hrs |
| Streptococcus pneumoniae ATCC 6303 | 50 -100 | luxuriant | 35 -100 | >=70 % | 35 -100 | 18 -24 hrs |
| Neisseria meningitidis ATCC 13090 | 50 -100 | luxuriant | 35 -100 | >=70 % | 35 -100 | 18 -24 hrs |
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle inorder to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Use before expiry date on the label.
Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (2,3).
Reference
- Murray P. R., Baron J. H., Pfaller M. A., Jorgensen J. H. and Yolken R. H., (Ed.), 2003, Manual of Clinical Microbiology, 8th Ed., American Society for Microbiology, Washington, D.C.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.
- Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | Casitose Soya Blood Agar Base |
|---|---|
| SKU | M1796 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Murray P. R., Baron J. H., Pfaller M. A., Jorgensen J. H. and Yolken R. H., (Ed.), 2003, Manual of Clinical Microbiology,8th Ed., American Society for Microbiology, Washington, D.C. |
| Customized Product Available | No |