Your enquiry has been submitted
Your enquiry has been submitted
Listeria Oxford Agar Base w/ 1.2% Agar
Intended Use:
Recommended for isolation of Listeria species from food sample in accordance with FDA BAM, 1998.
Composition**
| Ingredients | Gms / Litre |
|---|---|
| Peptone, special | 23.000 |
| Lithium Chloride | 15.000 |
| Sodium chloride | 5.000 |
| Corn Starch | 1.000 |
| Esculin | 1.000 |
| Ammonium Ferric Citrate | 0.500 |
| Agar | 12.000 |
Final pH (at 25°C): 7.2±0.1
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 28.75 grams in 500 ml purified/distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C and aseptically add the rehydrated contents of 1 vial of CM Selective Supplement, Modified (FD126F). Mix well before pouring into sterile Petri plates.
Principle And Interpretation
The genus Listeria contains six species; L. monocytogenes, L. innocua, L. seeligeri, L. welshimeri, L. ivanovii, and L. grayi. Listeria monocytogenes is the only species of the genus Listeria that is important as a human pathogen. The universal occurrence of L. monocytogenes in food (1,2,3) and the risk of contracting food-borne L. monocytogenes listeriosis has been thoroughly reviewed recently and L. monocytogenes has been detected in the food processing environment, such as on food contact surfaces and equipment(4). Other species; Listeria seeligeri, Listeria welshimeri and Listeria ivanovii have been related with animal diseases. L. ivanovii and L. monocytogenes are pathogenic for mice and other animals. However, only L. monocytogenes is commonly associated with human listeriosis. Listeriosis associated infection by L. ivanovii, and even L. seeligeri is extremely rare in humans (5).
The preferred standard methodology, and permitted alternative rapid methodologies, to be used for detection and isolation of Listeria monocytogenes are as follows. Presumptive contaminated food lots are sampled. Generally, sub-samples are composited if required by FDA field laboratory instructions. Analytical portions (25 g) are pre-enriched for Listeria species at 30°C for 4h in buffered Listeria Enrichment broth (BLEB) (M1578), equivalent to AOAC/IDF dairy products enrichment broth base containing sodium pyruvate (6,7,8). At the fourth hour of the incubation, the selective agents (acriflavin, 10 mg/L; sodium nalidixate, 40 mg/L; optional antifungal, e.g. cycloheximide 50 mg/L) are added. Incubation for selective enrichment is continued at 30° C for a total of 48 h. The enrichment culture is streaked at 24 and 48 h on one of the prescribed differential selective-agars in order to isolate Listeria species. The selective and differential media containing esculin and ferric citrate aids in Listeria isolation and detection based on esculin hydrolysis. Listeria Oxford Medium Base is based on the formulation described by Curtis et al (9) for the selective isolation of L. monocytogenes from food specimens, and is recommended by FDA BAM for the same purpose (10).
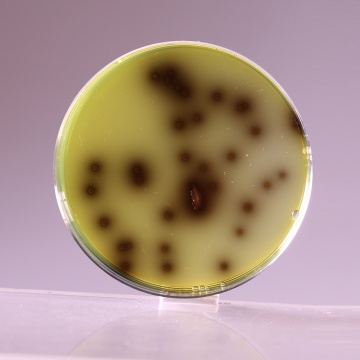
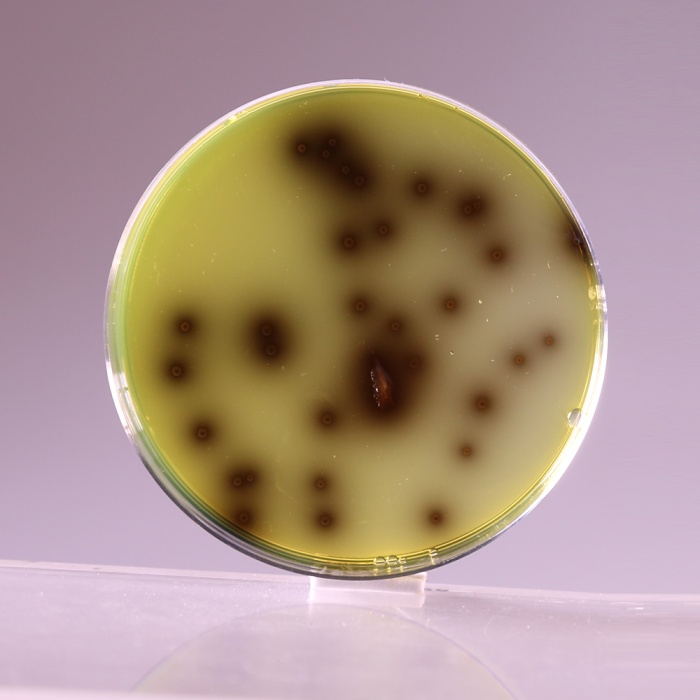
Peptone special serves as the source of essential nutrients to the organisms. Corn starch serves to neutralize the toxic metabolites formed. Lithium chloride and the antibiotics inhibit gram-negative bacteria and most gram-positive organisms but certain strains of Staphylococci may grow as esculin negative colonies. Colistin sulphate, moxalacatam and lithium chloride inhibit bacteria other than Listeria species. Alternatively CM Selective Supplement, Modified (FD126F) can be added which inhibits both gram-positive and gram-negative bacteria. L. monocytogenes hydrolyzes esculin to esculetin and dextrose. Esculetin reacts with ferric ions and produces black zones around the colonies.
Type of specimen
Food samples
Specimen Collection and Handling
For food and environmental samples selective enrichment is generally used (10,11,12). For isolation of Listeria from food (milk and milk products), 25 ml or 25 grams of sample is enriched in 225 ml of Buffered Listeria Enrichment Broth (M1578). Homogenize and mix carefully. Incubate for 4 hours at 30°C. After 4 hours add Selective agents (FD063I) containing nalidixic acid, cycloheximide, acriflavine hydrochloride is added to enhance selectivity. Incubate for 48 hours at 30°C. The enriched cultures are streaked onto Listeria Oxford medium Base at 24 and 48 hours. Incubate aerobically for 24-48 hours at 35°C. Take 5 esculin positive colonies that appear typical black coloured and inoculate onto Tryptone Soya Yeast Extract agar (M1214/M1214F). Incubate for 24-48 hours at 30°C and then confirm colonies using Henrys illumination light.
Warning and Precautions :
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets
Limitations :
- Further biochemical tests are needed for a final identification of the isolated organisms.
- Individual organisms differ in their growth requirement and may show variable growth patterns on the medium.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance Light yellow to dark yellow homogeneous free flowing powder
Gelling Firm, comparable with 1.2% Agar gel.
Colour and Clarity of prepared medium Dark amber coloured clear to slightly opalescent gel with a blue cast forms in Petri plates
Reaction Reaction of 5.75% w/v aqueous solution at 25°C. pH: 7.2±0.1
pH 7.10-7.30
Cultural Response
Cultural characteristics observed with added CM Selective Supplement, Modified (FD126F), after an incubation at 35°C for 24-48 hours.
| Organism | Inoculum (CFU) | Growth | Recovery | Esculin Hydrolysis |
|---|---|---|---|---|
| Bacillus subtilis ATCC 6633 (00003*) | >=104 | inhibited | 0% | |
| Enterococcus faecalis ATCC 29212 (00087*) | >=104 | inhibited | 0% | |
| Enterococcus hirae ATCC 10541 (00011*) | >=104 | inhibited | 0% | |
| Escherichia coli ATCC 25922 (00013*) | >=104 | inhibited | 0% | |
| Listeria monocytogenes ATCC 19111 (00020*) | 50-100 | luxuriant | >=50% | positive reaction, blackening of medium around the colony |
| Listeria monocytogenes ATCC 19112 | 50-100 | luxuriant | >=50% | positive reaction, blackening of medium around the colony |
| Listeria monocytogenes ATCC 19117 | 50-100 | luxuriant | >=50% | positive reaction, blackening of medium around the colony |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | 50-100 | good | 40-50% | negative reaction |
Key: (*) Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle inorder to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (13,14).
Reference
- Abou-Eleinin A.-A. M., Ryser E.T. and Donnelly C.W.,2000, Journal of Food Protection, 63:1208-1213.
- Twedt R. M., Hitchins A. D., and Prentice G. A., 1994. International Dairy Federation Method, J. AOAC INTERNATIONAL,77:395-402.
- US DHHS/FDA/CFSAN and USDA/FSIS. 2003.
- Entis P. and Lerner.I., 2000, J. Food Protection, 63:354-363.
- Bille J., Rocourt J., and Swaminathan B., 1999, Listeriae, Erysipelothrix, and Kurthia, pp. 295-314. In: Manual of Clinical Microbiology. 7th Edition. P. R.Murray (ed.). American Society for Microbiology, Washington, DC.
- AOAC Official Method 993.12., 2000, Listeria monocytogenes in Milk and Dairy Products, Selective Enrichment and Isolation Method (IDF Method).Chapter 17.10.01, pp. 138-139 In: Official Methods of Analysis of AOAC INTERNATIONAL. 17th Edition. W. Horwitz (ed.). Volume 1. Agricultural Chemicals, Contaminants and Drugs. AOAC INTERNATIONAL, Gaithersburg, MD.
- Hitchins A. D., and Duvall R. E., 2000, J. Food Protect. 63:1064-1070.
- Wang, S-Y. and Hitchins, A. D., 1994. J. Food Safety 14:259-27.
- Curtis G. D. W. MRG, King A. F., Griffin E. J., 1989, Lett. Appl. Microbiol.,8:95.
- FDA US. Bacteriological Analytical Manual. 8 ed. Gaithersburg, MD. : AOAC International; 1998.
- Asperger H., Heistinger H., Wagner M., Lehner A. and Brandl E., 1999. Microbiology, 16:419-431.
- Hayes P. S, Feeley J. L, Groves L. M, Ajello G. W and Fleming D. W.,1986, Appl Environ Microbiol. 51:438.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W.(2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | Listeria Oxford Agar Base w/ 1.2% Agar |
|---|---|
| SKU | M1145F |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1.US DHHS/FDA/CFSAN and USDA/FSIS. 2003.2.Abou-Eleinin A.-A. M., Ryser E.T. and Donnelly C.W.,2000, Journal of Food Protection, 63:1208-1213.3.Twedt R. M., Hitchins A. D., and Prentice G. A., 199 |
| Customized Product Available | No |