Your enquiry has been submitted
Your enquiry has been submitted
XLD Agar
Intended Use
Recommended for isolation and enumeration of Salmonella Typhi and other Salmonella species in accordance with FDA BAM, APHA.
Composition**
| Ingredients | FDA BAM Gms / Litre |
Ingredients | M031F Gms / Litre |
|---|---|---|---|
| Yeast extract | 3.000 | Yeast extract | 3.000 |
| L-Lysine | 5.000 | L-Lysine | 5.000 |
| Xylose | 3.750 | Xylose | 3.750 |
| Lactose | 7.500 | Lactose | 7.500 |
| Sucrose | 7.500 | Sucrose | 7.500 |
| Sodium desoxycholate | 2.500 | Sodium desoxycholate | 2.500 |
| Ferric ammonium citrate | 0.800 | Ferric ammonium citrate | 0.800 |
| Sodium thiosulphate | 6.800 | Sodium thiosulphate | 6.800 |
| Sodium Chloride | 5.000 | Sodium Chloride | 5.000 |
| Phenol red | 0.080 | Phenol red | 0.080 |
| Agar | 15.000 | Agar | 15.000 |
Final pH (at 25°C): 7.4±0.2
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 56.93 grams in 1000 ml purified/distilled water. Heat with frequent agitation until the medium boils. DO NOT AUTOCLAVE OR OVERHEAT. Transfer immediately to a water bath at 50°C. After cooling, pour into sterile Petri plates. It is advisable not to prepare large volumes that will require prolonged heating, thereby producing precipitate.
Note: Slight precipitation in the medium may occur, which is inheritant property of the medium, and does not affect the performance of the medium.
Principle And Interpretation
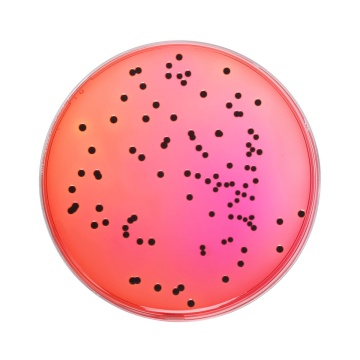
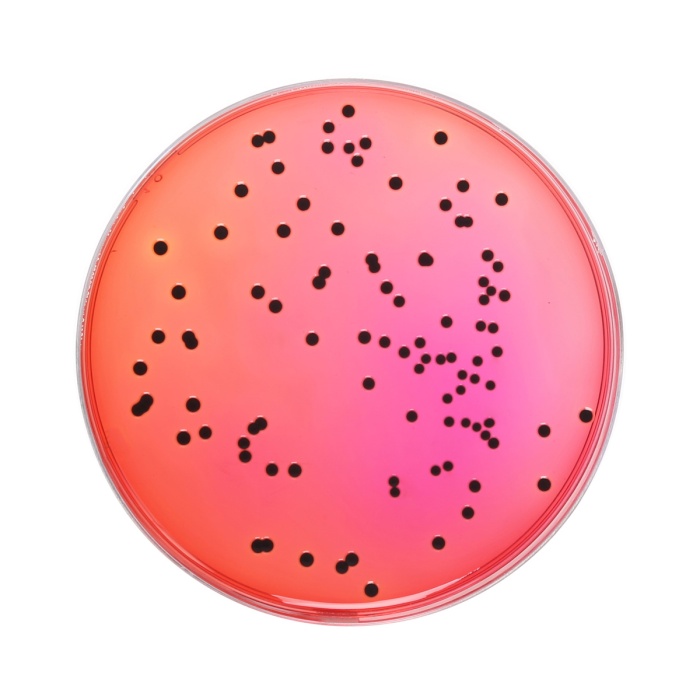
XLD Agar was formulated by Taylor (1) for the isolation and differentiation of enteric pathogens including Salmonella Typhi from other Salmonella species. The media has been recommended for the identification of Enterobacteriaceae (2) water and dairy products (3,4). This has also been recommended by FDA BAM, APHA for the selective isolation and identification of Salmonella from food specimens (5,6). XLD Agar exhibits increased selectivity and sensitivity as compared to other plating media such as SS Agar, EMB Agar and Bismuth Sulphite Agar (7,1). The medium contains yeast extract, which provides nitrogen and vitamins required for growth. Sodium chloride maintains the osmotic balance of the medium. Lysine is included to differentiate the Salmonella group from the non-pathogens. Salmonellae rapidly ferment xylose and exhaust the supply. Subsequently lysine is decarboxylated by the enzyme lysine decarboxylase to form amines with reversion to an alkaline pH. However, to prevent this reaction by lysine-positive coliforms, lactose and sucrose are added to produce acid in excess. Degradation of xylose, lactose and sucrose to acid causes phenol red indicator to change its colour to yellow. Bacteria that decarboxylate lysine to cadaverine can be recognized by the appearance of a red colouration around the colonies due to an increase in pH. These reactions can proceed simultaneously or successively, and this may cause the pH indicator to exhibit various shades of colour or it may change its colour from yellow to red on prolonged incubation. To add to the differentiating ability of the formulation, an H2S indicator system, consisting of sodium thiosulphate and ferric ammonium citrate, is included for the visualization of hydrogen sulphide produced, resulting in the formation of colonies with black centers. The non-pathogenic H2S producers do not decarboxylate lysine; therefore, the acid reaction produced by them prevents the blackening of the colonies. XLD Agar is both selective and differential medium. It utilizes sodium deoxycholate as the selective agent and therefore it is inhibitory to gram-positive microorganisms. Some Proteus strains may give red to yellow colouration with most colonies developing black centers, giving rise to false positive reactions. Non-enterics like Pseudomonas and Providencia may exhibit red colonies. S. Paratyphi A, S. Choleraesuis, S. Pullorum and S. Gallinarum may form red colonies without H2S, thus resembling Shigella species (8).
Type of specimen
Food samples
Specimen Collection and Handling
According to the BAM procedure (5), 25g of the food sample is pretreated with suitable diluents such as Lactose Broth (M1003) or Buffered Peptone Broth (M614) or Universal Pre-enrichment Broth (M1372F); depending upon the type and nature of the sample. Typically, for specimens with low microbial load, sample to broth ratio has been recommended to be 1:9. Inoculated broth is further incubated at 35 ± 0.2°C for 24 ± 2 hrs. In case of food samples with high microbial load, 0.1 ml of sample mixture is mixed with 10 ml of Tetrathionate broth (M032F) and incubated at 43 ± 0.2°C for 24 ± 2hrs. After incubation, 10 µl of the corresponding broth is inoculated on Xylose Lysine Deoxycholate Agar (M031F). After incubation period of 24 ± 2 hrs at 35°C, plates are checked for Salmonella colonies. Typical Salmonella colonies appear as pink to red colored with or without black centers. Many cultures of Salmonella may produce colonies with large, glossy black centers or may appear as almost completely black colonies. Atypically a few Salmonella cultures produce yellow colonies with or without black centers. Cultures identified using XLD agar are further confirmed through biochemical tests.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/ face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred to individual safety data sheets.
Limitations
- Slight precipitation in the medium may occur, which is inheritant property of the medium,and does not affect the performance of the medium.
- This medium is general purpose medium and may not support the growth of fastidious organisms.
- Some Proteus strains may give red to yellow colouration with most colonies developing black centers, giving rise to false positive reactions.
- Non-enterics like Pseudomonas and Providencia may exhibit red colonies.
- S. Paratyphi A, S.Choleraesuis, S. Pullorum and S. Gallinarum may form red colonies without H2S, thus resembling Shigella species.
- Atypical Salmonella species which are lactose positive and/or H2S negative should be confirmed by biochemical and serological tests.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Light yellow to pink homogeneous free flowing powder
Gelling: Firm, comparable with 1.5% Agar gel
Colour and Clarity of prepared medium: Red coloured clear to slightly opalescent gel forms in Petri plates
Reaction: Reaction of 5.69% w/v aqueous solution at 25°C. pH: 7.4±0.2
pH: 7.20-7.60
Cultural Response: Cultural characteristics observed after an incubation at 35-37°C for 22-26 hours.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of Colony |
|---|---|---|---|---|
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | >=50% | pink-red with black centres |
| Salmonella Abony NCTC 6017 | 50-100 | luxuriant | >=50% | pink-red with black centres |
| Salmonella Paratyphi A ATCC 9150 | 50-100 | good-luxuriant | >=50% | pink |
| Salmonella Paratyphi B ATCC 8759 | 50-100 | good-luxuriant | >=50% | pink-red with black centres |
| Salmonella Enteritidis ATCC 13076 (00030*) | 50-100 | good-luxuriant | >=50% | red with black centres |
| Salmonella Typhi ATCC 6539 | 50-100 | good-luxuriant | >=50% | pink-red with black centres |
Key
*Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (9,10).
Reference
- Taylor, W. L. and Schelhart, B. 1969. Appl. Microbiol., 18: 393-395.
- Chadwick, P., Delisle, G. H.. and Byer, M. 1974. Can. J. Microbiol., 20: 1653-1664.
- Andrew, E. D., Rice, E. W., Greenberg, A. E. and S, Clesceri L. 2005. APHA Washington, D.C.
- Wehr, H.M. and Frank, J.H. 2004. Standard Methods for the Examination of Dairy Products. 17 ed.
- FDA Bacteriological Analytical Manual, 2017, M179, AOAC, Washington, DC.
- Salfinger Y., and Tortorello M.L., 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Dunn, C. and Martin, W. J. 1971. Appl. Microbiol., 22: 17-22.
- MacFaddin, J. F. 1985. Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria. vol. 1. Baltimore: Williams and Wilkins.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | XLD Agar |
|---|---|
| SKU | M031F |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Taylor W. L., 1965, Am. J. Clin. Pathol., 44:471-475. 2.Taylor W. L. and Harris B., 1965, Am. J. Clin. Pathol., 44:476. 3.Taylor W. L. and Harris B., 1967, Am. J. Clin. Pathol., 48:350. 4.Taylor W. L. and Schelhart B., 1967, Am. J. Clin. Pathol., 48:356. 5.Taylor W. L. and Schelhart B., 1968, Am. J. Clin. Pathol., 16:1387. 6.Taylor W. L. and Schelhart B., 1969, Appl. Microbiol., 18.393-395. 7.Chadwick P., Delisle G. H and Byer M., 1974, Can. J. Microbiol., 20, 1653-1664. 8.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 9.Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water andWastewater, 23rd ed., APHA, Washington, D.C. 10.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 11. Williams H., (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed., AOAC,Washington, D.C.1 2.FDA Bacteriological Analytical Manual, 2005, 18th Ed., AOAC, Washington, D.C. 13.Dunn C. and Martin W. J., 1971, Appl. Microbiol., 22, 17-22.1 4.Rollender M. A., Beckford O., Belsky R. D and Kostroff B. 1969, Am. J. Clin. Pathol., 51, 284-286. 15.Taylor W. L. and Schelhart B., 1969, Appl. Micro. 18, 1387-1392. 16.MacCarthy M. D., 1966, N. Z. J. Med. Lab. Technol., 20, 127-131. 17.Isenberg H. D., Kominos S., and Sigeal M., 1969, Appl Microbiol., 18, 656-659.1 20.Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.21. Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |