Your enquiry has been submitted
Your enquiry has been submitted
Cetrimide Agar, Granulated
Intended use
Recommended for the selective isolation of Pseudomonas aeruginosa from pharmaceutical products in accordance with the microbial limit testing by harmonized methodology of USP/EP/BP/JP.
Composition**
| Ingredients | g/L |
|---|---|
| Gelatin peptone # | 20.000 |
| Magnesium chloride | 1.400 |
| Dipotassium sulphate | 10.000 |
| Cetrimide | 0.300 |
| Agar | 13.600 |
| pH after sterilization (at 25°C) | 7.2±0.2 |
**Formula adjusted, standardized to suit performance parameters
# Pancreatic digest of gelatin
Directions
Suspend 45.3 grams in 1000 ml purified/distilled water containing 10 ml glycerin/glycerol. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes or as per validated cycle. Cool to 45-50°C. Mix well and pour into sterile Petri plates.
Principle And Interpretation
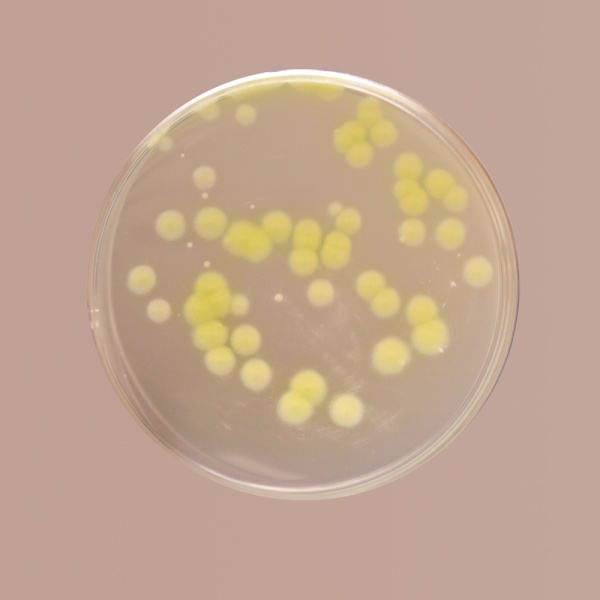
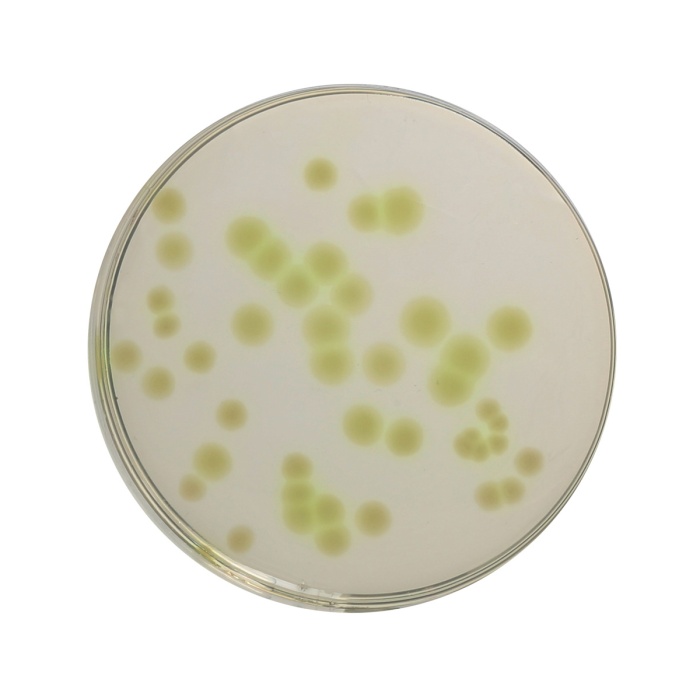
Cetrimide Agar was described by King et al (1). This media formulation is in accordance with the harmonized method of USP/EP/BP/JP (2-5). It is used as a selective medium for the isolation of Pseudomonas aeruginosa from pharmaceutical products. This medium is also used for microbial limit testing for non- sterile products. Lowburry first reported the use of cetrimide as an agent for selective isolation of Pseudomonas (6). This medium is also used for determining the ability of an organism to produce fluorescein and pyocyanin. Cetrimide (N-acetyl-N,N,N- trimethylammonium bromide) is incorporated in the medium to inhibit bacteria other than Pseudomonas aeruginosa. This compound a cationic detergent acts as a quaternary ammonium compound, which causes nitrogen and phosphorus to be released from bacterial cells other than Pseudomonas aeruginosa. Magnesium chloride and potassium sulphate incorporated in the medium enhances the production of pigment pyocyanin, which is a blue-green pigment, diffusing into the medium. This improves detection of Pseudomonas on this medium. Presence of magnesium ions can also neutralizes EDTA, if present in the sample. Gelatin peptone provides the essential nutrients for growth of Pseudomonas, while glycerin serves as slow and continuous carbon source for the growing cell.
For the isolation of Pseudomonas aeruginosa, plates of Cetrimide Agar should be inoculated from non-selective medium such as Soybean Casein Digest Medium (MH011). If the count is high the test sample can be directly inoculated onto this medium. Pseudomonas aeruginosa colonies may appear pigmented greenish (under uv light also).
Type of specimen
Pharmaceutical samples
Specimen Collection and Handling
For pharmaceutical samples, follow appropriate techniques for sample collection, processing as per guidelines (2-5). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions:
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Individual organisms differ in their growth requirement and may show variable growth patterns on the medium.
- Each lot of the medium has been tested for the organisms specified on the COA. It is recommended to users to validate the medium for any specific microorganism other than mentioned in the COA based on the user's unique requirement.
- This medium is a selective medium, some strains may show poor growth as cetrimide is highly toxic.
- Further biochemical tests must be carried out for complete identification.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance
Cream to yellow coloured granular medium.
Gelling
Firm, comparable with 1.36% Agar gel
Colour and Clarity of prepared medium
Light amber coloured opalescent gel with a slight precipitate forms in Petri plates
pH
7.00-7.40
Growth Promotion Test
Growth Promotion is carried out in accordance with the harmonized method of USP/EP/BP/JP. Cultural response was observed after an incubation at 30-35°C for specified time. Recovery rate is considered as 100% for bacteria growth on Soybean Casein Digest Agar.
Growth promoting properties
Growth of microorganism comparable to that previously obtained with previously tested and approved lot of medium occurs at the specified temperature for not more than the shortest period of time specified inoculating <=100 cfu (at 30-35°C for <=18 hours).
Inhibitory properties
No growth of the test microorganism occurs for the specified temp for not less than longest period of time specified inoculating >=100 cfu (at least 100 cfu) (at 30-35°C for >= 72 hours).
Cultural Response
Cultural characteristics observed after incubation at 30-35 °C for 18-72 hours. Recovery rate is considered as 100% for bacteria growth on Soyabean Casein Digest Agar.
| Organism | Inoculum (CFU) | Growth | Observed Lot value (CFU) | Recovery | Incubation temperature | Incubation period |
|---|---|---|---|---|---|---|
| Growth promoting | ||||||
| ^Pseudomonas paraeruginosa ATCC 9027 (00026*) | 50-100 | luxuriant | 25-100 | >=50% | 30-35 °C | <=18 hrs |
| Inhibitory | ||||||
| Escherichia coli ATCC 8739 (00012*) | >=103 | inhibited | 0 | 0% | 30-35 °C | >=72 hrs |
| Additional Microbiological testing | ||||||
| Pseudomonas aeruginosa ATCC 27853(00025*) | 50-100 | luxuriant | 25-100 | >=50% | 30-35 °C | 18-24 hrs |
| Pseudomonas aeruginosa ATCC 25668 (00114*) | 50-100 | luxuriant | 25-100 | >=50% | 30-35 °C | 18-24 hrs |
| Stenotrophomonas maltophila ATCC 13637 | >=103 | inhibited | 0 | 0% | 30-35 °C | >=72 hrs |
| Escherichia coli ATCC 25922 (00013*) | >=103 | inhibited | 0 | 0% | 30-35 °C | >=72 hrs |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | >=103 | inhibited | 0 | 0% | 30-35 °C | >=72 hrs |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | >=103 | inhibited | 0 | 0% | 30-35 °C | >=72 hrs |
| Salmonella Typhimurium ATCC 14028 (00031*) | >=103 | inhibited | 0 | 0% | 30-35 °C | >=72 hrs |
| Proteus mirabilis ATCC 29906 (00023*) | >=103 | inhibited | 0 | 0% | 30-35 °C | >=72 hrs |
Key: (*) Corresponding WDCM numbers, ^ Formerly known as Pseudomonas aeruginosa
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (7,8).
| Product Name | Cetrimide Agar, Granulated |
|---|---|
| SKU | GMH024 |
| Product Type | Granulated |
| Physical Form | Granular |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1.King, Ward and Raney, 1954, J. Lab. Clin. Med., 44:301. 2.Lowbury, 1951, J. Clin. Pathol., 4:66. 3.Lowbury and Collins, 1955, J. Clin. Pathol., 8:474.Brown and Lowbury, 1965, J. Clin. Pathol., 18:752. 5.Murray P. R., Baron J. H., Pfaller M. A., Jorgensen J. H. and YolkenR. H., (Ed.), 2003, Manual of Clinical Microbiology,8th Ed., American Society for Microbiology, Washington, D.C. 6.USFDA Bacteriological Analytical Manual, 2005, 18th Ed., AOAC, Washington, DC. 7.Forbes B. A., Sahm A. S. and Weissfeld D. F., Bailey & Scotts Diagnostic Microbiology, 10th Ed., 1998, Mosby, Inc., St.Louis, Mo. 8.Williams, (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed., AOAC,Washington, D.C.9.MacFaddin J. F., 1985, Media for Isolation-Cultivation-Identification -Maintenance of Medical Bacteria, Vol. I, Williamsand Wilkins, Baltimore. 10.Goto and Enomoto, 1970, Jpn. J. Microbiol., 14:65. 11.Greenberg A. E., Clesceri L. S. and Eaton A. D., (Eds.), 2005, Standard Methods for the Examination of Water andWastewater, 21st ed., APHA, Washington, D.C. 12.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition. 13.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |