Your enquiry has been submitted
Your enquiry has been submitted
Casein Soyabean Digest Broth, Granulated, Sterile (Soyabean Casein Digest Medium, Granulated, Sterile)
Intended Use
It is a irradiated sterile powder recommended for evaluation of sterility in manufacturing process.
Composition
| Ingredients | Gms / Litre |
|---|---|
| Tryptone # | 17.000 |
| Soya peptone ## | 3.000 |
| Sodium chloride | 5.000 |
| Dipotassium hydrogen phosphate | 2.500 |
| Glucose monohydrate | 2.500 |
| Final pH (at 25°C) | 7.3±0.2 |
**Formula adjusted, standardized to suit performance parameters
# Pancreatic digest of casein,## Papaic digest of soybean (soyabean)
Directions
Sterile powder can be used directly for the evaluation of sterility in manufacturing process. For sterile liquid medium aseptically add 29.77 grams (the equivalent weight of dehydrated medium per litre) in 1000 ml sterile distilled water. Heat if necessary to dissolve the medium completely. Excessive heating is detrimental. Dispense aseptically in sterile flasks or tubes as desired. Recommended NOT TO AUTOCLAVE OR OVERHEAT.
Principle And Interpretation
Soyabean Casein Digest Medium is recommended as a sterility testing medium in accordance with the harmonized method of USP/EP/BP/JP/IP (1-5). It is used for the sensitivity testing of antimicrobial agents by the tube dilution method (6). It is also employed in diagnostic research in microbiology.
The combination of tryptone and soya peptone makes this medium nutritious by providing amino acids and long chain peptides for the growth of microorganisms. Natural sugars in soybean promote growth of fastidious organism. Glucose is the fermentable source of carbon and dibasic potassium phosphate serves as the buffer in the medium. Sodium chloride maintains the osmotic balance of the medium
This medium is recommended for sterility checking and for studying total aerobic microbial count in verification of microbiological testing procedures employed for sterility checking.
Type of specimen
Pharmaceutical samples
Specimen Collection and Handling
Forpharmaceuticalsamples,followappropriatetechniquesforsamplecollection,processing as per pharmaceutical guidelines (1-5). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Biochemical characterization is necessary to be performed on colonies from pure cultures for further identification.
- This medium is general purpose medium and may not support the growth of fastidious organisms.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance
Cream to yellow coloured granular medium
Colour and Clarity of prepared medium
Light yellow coloured clear solution without any precipitate.
Reaction
pH of 2.98% w/v aqueous solution at 25°C (after sterilization). pH: 7.3±0.2
pH
7.10-7.50
Sterility Testing
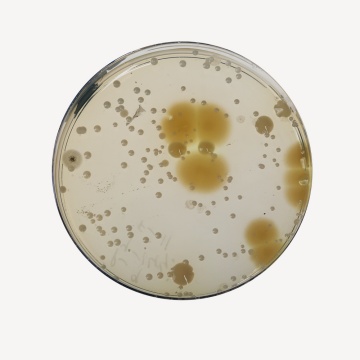
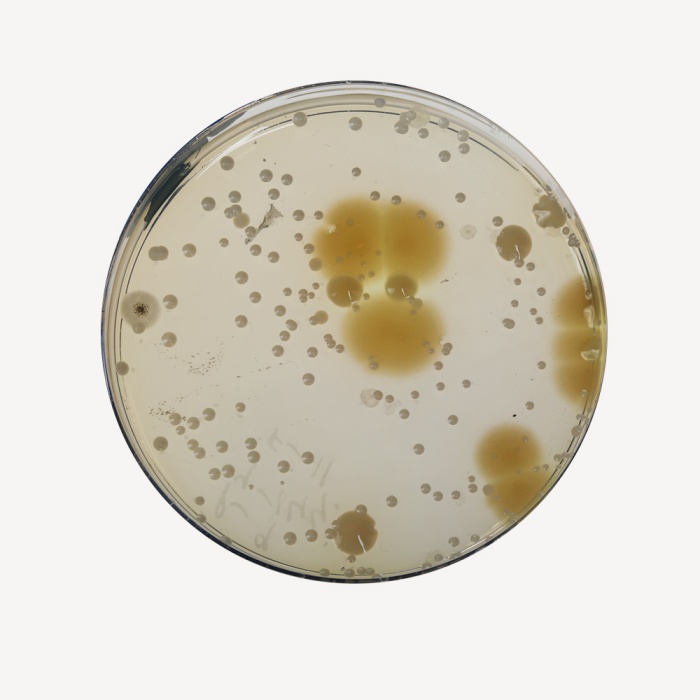
No growth is observed after 14 days for Bacteria at 30-35°C and for fungi at 20-25°C.
Test for Mycoplasma
None detected.
Stability test
Light yellow coloured clear solution without any precipitation or sedimentation at room temperature for 7 days.
Growth Promotion Test
In accordance with the harmonized method of USP/EP/BP/IP.
Growth promoting properties
Clearly visible growth of microorganism comparable to that previously obtained with previously tested and approved lot of medium occurs at the specified temperature for not more than the shortest period of time specified inoculating <=100 cfu(at 30-35°C for 18-24 hours).
Sterility Testing + Validation
The medium is tested with suitable strains of microorganisms inoculating <=100cfu and incubating at 20-25°C for not more than 3 days in case of bacteria and not more than 5 days in case of fungi.
Cultural Response
Growth promoting
| Organism | Inoculum (CFU) | Growth | Incubation period | Incubation temperature |
|---|---|---|---|---|
| Salmonella Abony NCTC 6017 (00029*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Pseudomonas aeruginosa ATCC 27853 (00025*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Bacillus subtilis subsp. spizizenii ATCC 6633 (00003*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| $ Kocuria rhizophila ATCC 9341 | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Escherichia coli ATCC 8739 (00012*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Pseudomonas aeruginosa ATCC 9027 (00026*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | 50-100 | luxuriant | 18-24 hrs | 30-35 °C |
Sterility Testing- Growth promotion+Validation
| Organism | Inoculum | Growth | Incubation | Incubation temperature |
|---|---|---|---|---|
| Candida albicans ATCC 2091 (00055*) | 50-100 | luxuriant | <=5 d | 20-25 °C |
| Candida albicans ATCC 10231 (00054*) | 50-100 | luxuriant | <=5 d | 20-25 °C |
| #Aspergillus brasiliensis ATCC 16404 (00053*) | 50-100 | luxuriant | <=5 d | 20-25 °C |
| Salmonella Abony NCTC 6017 (00029*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Pseudomonas aeruginosa ATCC 27853 (00025*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| $ Kocuria rhizophila ATCC 9341 | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Escherichia coli ATCC 8739 (00012*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Pseudomonas aeruginosa ATCC 9027 (00026*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Bacillus subtilis subsp. spizizenii ATCC 6633 (00003*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | 50-100 | luxuriant | <=3 d | 20-25 °C |
Key: (#) Formerly known as Aspergillus niger, $- Formerly known as Micrococcus luteus (*) Corresponding WDCM numbers
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 15-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (7,8).
| Product Name | Casein Soyabean Digest Broth, Granulated, Sterile (Soyabean Casein Digest Medium, Granulated, Sterile) |
|---|---|
| SKU | GMH011G |
| Product Type | Granulated |
| Physical Form | Sterile Granular |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. MacFaddin J. F., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. 1, Williams& Wilkins, Baltimore, M.d. 2.The United States Pharmacopeia, 2018, USP 41/NF36, The United States Pharmacopeial Convention, Rockville, MD. 3.Indian Pharmacopeia, 2018, Govt. of India, Ministry of Health and Family Welfare, New Delhi, India. 4.Forbes B. A., Sahm D. F. and Weissfeld A. S., 1998, Bailey & Scotts Diagnostic Microbiology, 10th Ed., Mosby, Inc. St.Louis, Mo. 5.Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition. 6.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |