Your enquiry has been submitted
Your enquiry has been submitted
HiEncap™ MacConkey Agar w/0.15% Bile Salts, CV and NaCl
Intended Use
Recommended for selective isolation and differentiation of coliform organisms and other enteric pathogens.
Composition
| Ingredients | Gms/Litre |
|---|---|
| Gelatin peptone | 17.000 |
| Tryptone | 1.500 |
| Peptone | 1.500 |
| Lactose | 10.000 |
| Bile salts | 1.500 |
| Sodium chloride | 5.000 |
| Neutral Red | 0.030 |
| Crystal violet | 0.004 |
| Agar | 15.000 |
| Final pH (at 25°C) | 7.1±0.2 |
Formula adjusted, standardized to suit performance parameters
Directions
Each capsule contains 12.88 grams of media. Suspend 1 capsule in 250ml (4 capsules in 1000 ml) distilled or purified water. Heat to boiling to dissolve the agar completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Avoid overheating. Cool to 45 - 50°C.Mix well and pour into sterile Petri plates. The surface of the medium should be dry when inoculated.
Principle And Interpretation
MacConkey agars are slightly selective and differential plating media mainly used for the detection and isolation of gram-negative organisms from clinical (10), dairy (14), food (11,5), water (1), pharmaceutical (3,12) and industrial sources (15). It is also recommended for the selection and recovery of the Enterobacteriaceae and related enteric gram-negative bacilli. USP recommends this medium for use in the performance of Microbial Limit Tests (12).
These agar media are selective since the concentration of bile salts, which inhibit gram-positive microorganisms, is low in comparison with other enteric plating media. The medium EC081CCL, which corresponds with, that recommended by APHA can be used for the direct plating of water samples for coliform bacilli, for the examination of food samples for food poisoning organisms (11) and for the isolation of Salmonella and Shigella species in cheese (14). Other than that this medium is also used for count of coli-aerogenes bacteria in cattle and sheep faeces (9), the count of coli-aerogenes and non-lactose fermenters in poultry carcasses (2), bacterial counts on irradiated canned minced chicken (13) and the recognition of coli-aerogenes bacteria during investigations on the genus Aeromonas (4).
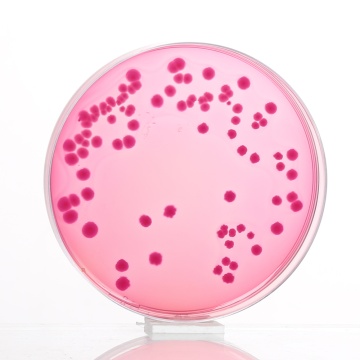
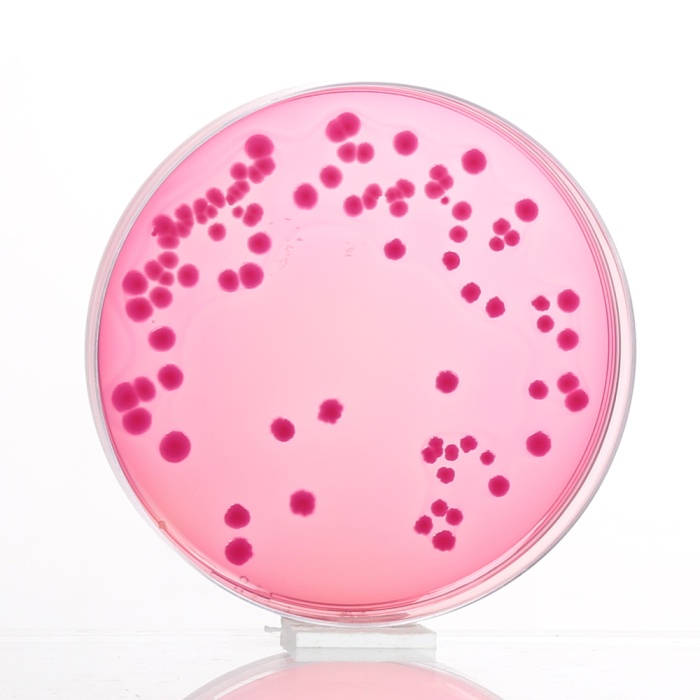
MacConkey Agar is the earliest selective and differential medium for cultivation of enteric microorganisms from a variety of clinical specimens (7,8). The original medium contains protein, bile salts, sodium chloride and two dyes. The selective action of this medium is attributed to crystal violet and bile salts, which are inhibitory to most species of gram-positive bacteria. Gram-negative bacteria usually grow well on the medium and are differentiated by their ability to ferment lactose. Lactose-fermenting strains grow as red or pink colonies and may be surrounded by a zone of acid precipitated bile. The red colour is due to production of acid from lactose, absorption of neutral red and a subsequent colour change of the dye when the pH of medium falls below 6.8. Lactose non-fermenting strains, such as Shigella and Salmonella are colourless, transparent and typically do not alter appearance of the medium.
Peptones are sources of nitrogen and other nutrients. Lactose is a fermentable carbohydrate, bile salts and crystal violet are selective agents that inhibit growth of gram-positive organisms. Neutral red is the pH indicator dye.
Type of specimen
Clinical - faeces, urine and other pathological material, Foodstuffs and dairy samples, Water samples, Pharmaceutical samples.
Specimen Collection and Handling
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (6,10). For food and dairy samples, follow appropriate techniques for sample collection and processing as per guidelines (5,11,14.). For water samples, follow appropriate techniques for sample collection, processing as per guidelines and local standards.(1) For pharmaceutical samples, follow appropriate techniques for sample collection, processing as per guidelines.(3,12) After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
In Vitro diagnostic use. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Though the medium is recommended for selective isolation, further biochemical and serological testing must be carried out for further confirmation.
- The surface of the medium should be dry when inoculated.
Performance and Evaluation
Performace of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Gelatin capsule containing light yellow to pink coloured granulated media.
Gelling: Firm comparable with 1.5% Agar gel.
Colour and Clarity of prepared medium: Red with purplish tinge coloured clear to slightly opalescent gel forms in Petri plates.
Quantity: Each capsule contains 12.88 grams of medium sufficient for 250 ml media
Reaction: Reaction of 4.95% w/v aqueous solution at 25°C. pH: 7.1±0.2
pH: 6.90-7.30
Cultural Response
Cultural response was observed after an incubation at 30-35°C for 18-72 hours. Recovery rate is considered as 100% for bacteria growth on Soybean Casein Digest Agar.
| Organism | Growth | Inoculum (CFU) | Recovery | Colour of colony | Incubation temperature | Incubation period |
|---|---|---|---|---|---|---|
| Salmonella Typhi ATCC 6539 | luxuriant | 50-100 | >=50% | colourless | 30-35 °C | 25-100 |
| Salmonella Enteritidis ATCC 13076 (00030*) | luxuriant | 50-100 | >=50% | colourless | 30-35 °C | 25-100 |
| Salmonella Paratyphi A ATCC 9150 | luxuriant | 50-100 | >=50% | colourless | 30-35 °C | 25-100 |
| Salmonella Paratyphi B ATCC 8759 | luxuriant | 50-100 | >=50% | colourless | 30-35 °C | 25-100 |
| Salmonella Abony NCTC 6017 | luxuriant | 50-100 | >=50% | colourless | 30-35 °C | 25-100 |
| Proteus vulgaris ATCC 13315 | luxuriant | 50-100 | >=50% | colourless | 30-35 °C | 25-100 |
| Staphylococcus epidermidis ATCC 12228 (00036*) | inhibited | >=104 | 0% | 30-35 °C | >=24 hrs | |
| Salmonella Typhimurium ATCC 14028 (00031*) | luxuriant | 50-100 | >=50% | colourless | 30-35 °C | 25-100 |
| Corynebacterium diphtheriae type gravis | inhibited | >=104 | 0% | 30-35 °C | >=24 hrs | |
| Escherichia coli ATCC 8739 (00012*) | luxuriant | 50-100 | >=50% | pink-red with bile precipitate | 30-35 °C | 25-100 |
| #Klebsiella aerogenes ATCC 13048 (00175*) | luxuriant | 50-100 | >=50% | pink to red | 30-35 °C | 25-100 |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | inhibited | >=104 | 0% | 30-35 °C | >=24 hrs | |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | inhibited | >=104 | 0% | 30-35 °C | >=24 hrs | |
| Shigella flexneri ATCC 12022 (00126*) | fair to good | 50-100 | 30-40% | colourless | 30-35 °C | 15-40 |
| Escherichia coli ATCC 25922 (00013*) | luxuriant | 50-100 | >=50% | pink to red with bile precipitate | 30-35 °C | 25-100 |
| Escherichia coli NCTC 9002 | luxuriant | 50-100 | >=50% | pink to red with bile precipitate | 30-35 °C | 25-100 |
| Enterococcus faecalis ATCC 29212 (00087*) | fair to good | 50-100 | 30-40% | colourless to pale pink | 30-35 °C | 15-40 |
Key :- * Corresponding WDCM numbers # Formerly known as Enterobacter aerogenes
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (6,10).
| Product Name | HiEncap™ MacConkey Agar w/0.15% Bile Salts, CV and NaCl |
|---|---|
| SKU | EC081CCL |
| Application/ Industry | Clinical, Food, Dairy, Water |
| Product Type | HiEncap™ |
| Physical Form | Granulated Media in Gelatin Capsule |
| Origin | Animal |
| Packaging type | HDPE Jar |
| References | 1.Murray P. R, Baron E, J., Jorgensen J. H., Pfaller M. A., Yolken R. H., (Eds.), 8th Ed., 2003, Manual of Clinical Microbiology, ASM, Washington, D.C. 2. Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed., APHA Inc., Washington, D.C. 3. Downes F. P. and Ito K., (Eds.), 2001, Compendium of Methods for the Microbiological Examination of Foods, 4th Ed., APHA, Washington, D.C. 4. FDA Bacteriological Analytical Manual, 2005, 18th Ed., AOAC, Washington, D.C.5.Eaton A. D., Clesceri L. S. and Greenberg A. W.,(Eds.), 2005, Standard Methods for the Examination of Water and Wastewater, 21st Ed., APHA, Washington, D.C.6.The United States Pharmacopoeia, 2009, The United States Pharmacopeial Convention, Rockville, M.D.7.Williams, (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed., AOAC,Washington, D.C8.Medrek T. F and Barnes Ella M., 1962, J. Appl. Bacteriol., 25(2),159-1689.Barnes Ella M. and Shrimpton D. H., 1957, J. Appl. Bacteriol., 20(2),273-285.10.Thornley Margaret J., 1957, J. Appl. Bacteriol., 20(2), 273-285.11.Eddy B. P., 1960, J. Appl. Bacteriol., 23(2).216-249.1 2. MacConkey A., 1905, J. Hyg., 5:33 3. 1 3. MacConkey A., 1900, The Lancet, ii:20.1 4. British Pharmacopoeia, 2009, The Stationery office British Pharmacopoeia. |