Your enquiry has been submitted
Your enquiry has been submitted
Slanetz and Bartley HiVeg® Medium
Intended Use
Slanetz and Bartley HiVeg® Medium is recommended for detection and enumeration of faecal Enterococci by membrane filtration technique.
Product Profile
This product offers a choice between vegetable-based and animal-based components:
| Vegetable based (Code MV)© | Animal based (Code M) |
|---|---|
| MV612 | M612 |
| HiVeg® hydrolysate No. 1 | Tryptose |
Composition
| Ingredients | Grams/Litre |
|---|---|
| HiVeg® hydrolysate No. 1 | 20.0 |
| Yeast extract | 5.0 |
| Dextrose | 2.0 |
| Disodium phosphate | 4.0 |
| Sodium azide | 0.4 |
| 2,3,5-Triphenyl tetrazolium chloride | 0.1 |
| Agar | 15.0 |
| Final pH (at 25°C) | 7.2± 0.2 |
** Formula adjusted, standardized to suit performance parameters.
Directions
Suspend 46.5 grams in 1000 ml distilled water. Heat to boiling to dissolve the medium completely. DO NOT AUTOCLAVE OR OVERHEAT. Excessive heating is detrimental.
Warning: Sodium azide has a tendency to form explosive metal-azides with plumbing materials. It is advisable to use enough water to flush off the disposables.
Principle and Interpretation
This medium is prepared by completely replacing animal based peptones with vegetable peptones making the medium free of BSE/TSE risks. Slanetz and Bartley HiVeg® Medium is the modification of Slanetz and Bartley Medium originally devised by Slanetz and Bartley (1) for the detection and enumeration of Enterococci by membrane filtration technique. Slanetz and Bartley HiVeg® Medium like the conventional medium, can be also used as a direct plating medium (2, 3).
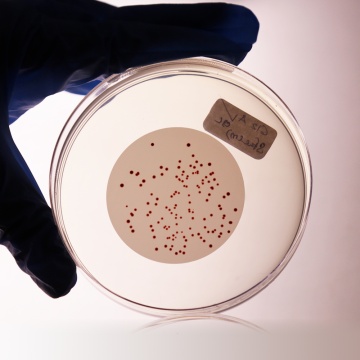
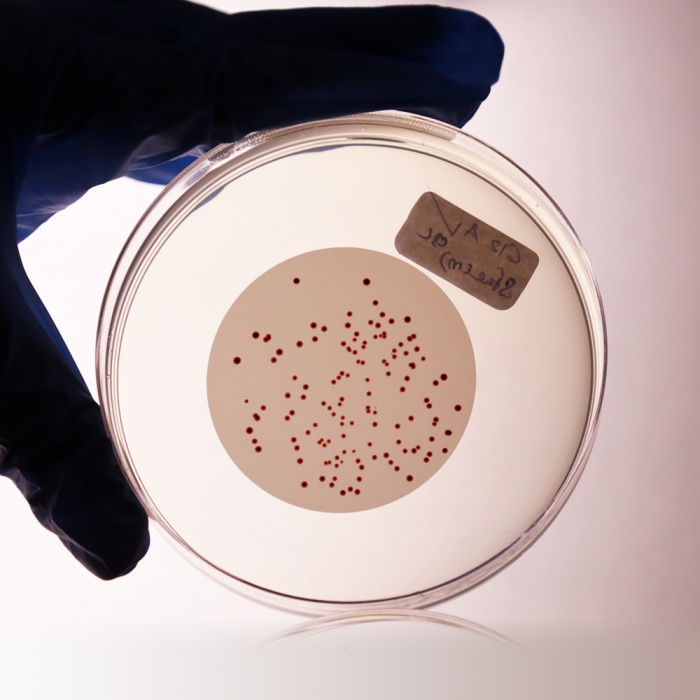
The medium is highly selective for Enterococci. Sodium azide has inhibitory effect on gram-negative organisms. Triphenyl Tetrazolium Chloride is reduced to insoluble formazan inside the bacterial cell forming dark red-coloured colonies. When the medium is incubated at higher temperature (44-45°C), all red or maroon colonies can be considered as presumptive Enterococci (4,5).
Reconstitution
Quantity on preparation (500g): 10.75 L
pH (25°C): 7.2± 0.2
Supplement: None
Sterilization: Boiling (DO NOT AUTOCLAVE)
Storage: Dry Medium - Below 30°C, Prepared Medium 2 - 8°C.
Usage Instructions
Water is filtered through a membrane filter which is then placed on the surface of the Slanetz and Bartley HiVeg® Medium plates and incubated at 35°C for 4 hours and then at 44-45°C for 44-48 hours. Red or maroon colonies are counted as Enterococci. Although incubation at 35°C yields a higher count, it allows the growth of organism which do not conform to the definition of Enterococci. Incubation at 44-45°C has a selective effect and produces fewer false - positives. The preliminary incubation at 35°C helps for the recovery of stressed organisms. Not all the species reduce TTC, hence pale colonies also should be considered.
Food samples are homogenized and diluted with physiological saline so as to give 15-150 colonies on each petri plate. Homogenates or dilutions are spread on agar surface and incubated at 35°C for 48 hours. Pink or dark red colonies with a narrow whitish border are counted (3).
Quality Control
Appearance of powder: Light yellow coloured, may have slightly greenish tinge, homogeneous, free flowing powder.
Gelling: Firm, comparable with 1.5% Agar gel.
Colour and Clarity: Light yellow coloured, slightly opalescent gel forms in petri plates.
Reaction: Reaction of 4.65% w/v aqueous solution is pH 7.2± 0.2 at 25°C.
Cultural Response
Cultural characteristics observed after an incubation at 44-45°C for 44-48 hours.
| Organisms (ATCC) | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| Enterococcus faecalis (29212) | 102-103 | luxuriant | >50% | red or maroon |
| Escherichia coli (25922) | 102-103 | inhibited | 0% | - |
| Product Name | Slanetz and Bartley HiVeg® Medium |
|---|---|
| SKU | MV612 |
| Product Type | HiVeg™ |
| Physical Form | Powder |
| Origin | Animal Free (Veg) |
| Packaging type | HDPE |
| References | 1. Slanetz L. W. and Bartley C.H., 1957, J. Bact., 74:591. 2.Burkwall M.K. and Hartman P.A., 1964, Appl. Microbiol., 12:18. 3.Nordic Committee on Food Analysis, 1968, Leaflet 68. 4.Taylor E.W. and Burman N.P., 1964, J. Appl. Bact., 27:294. 5.Mead G.C., 1966, Proc. Soc. Wat. Treat. Exam., 15:207. 6.Department of Health and Social Security, 1982, Report 71, HMSO, London. 7.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C. 8.Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water andWastewater, 23rd ed., APHA, Washington, D.C.9.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition.10.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |