Your enquiry has been submitted
Your enquiry has been submitted
Hektoen Enteric HiVeg® Agar
Intended Use
Hektoen Enteric HiVeg® Agar is recommended for differential and selective isolation of Shigella and Salmonella species from enteric pathological specimens.
Product Profile
Vegetable based (Code MV)
- MV467
- HiVeg® peptone No. 3
- Synthetic detergent No.l
Animal based (Code M)
- M467
- Proteose peptone
- Bile salts mixture
Composition
| Ingredients | Grams/Litre |
|---|---|
| HiVeg® peptone No.3 | 19.00 |
| Yeast extract | 3.00 |
| Lactose | 12.00 |
| Sucrose | 12.00 |
| Salicin | 2.00 |
| Synthetic detergent No. I | 2.00 |
| Sodium chloride | 5.00 |
| Sodium thiosulphate | 5.00 |
| Ferric ammonium citrate | 1.50 |
| Acid fuchsin | 0.10 |
| Bromo thymol blue | 0.065 |
| Agar | 15.00 |
Final pH (at 25°C) 7.5± 0.2
Formula adjusted, standardized to suit performance parameters
Reconstitution
76.67 g/l
| Quantity on preparation | Volume |
|---|---|
| (500g) | 6.52 L |
| (100g) | 1.3 L |
pH (25°C)
7.5± 0.2
Supplement
None
Sterilization
Boiling (DO NOT AUTOCLAVE).
Storage
Dry Medium - Below 30°C, Prepared Medium 2 - 8°C.
Directions
Suspend 76.67 grams in 1000 ml distilled water. Heat to boiling to dissolve the medium completely. DO NOT AUTOCLAVE.
Principle and Interpretation
Hektoen Enteric HiVeg® Agar is formulated by replacing animal base Proteose peptone by HiVeg® peptone No. 3 making it free from BSE/ TSE. Hektoen Enteric HiVeg® Agar is the modification of Hektoen Enteric Agar which was formulated by King and Metzger (1) and recommended by APHA (2). The increased concentration of carbohydrate and vegetable peptone helps to reduce the inhibitory effect of synthetic detergent and the indicators allow good growth of Salmonella and Shigella species while inhibiting the normal intestinal flora. The medium contains three carbohydrates lactose, sucrose and salicin for optional differentiation of enteric pathogens. The higher lactose concentration aids in the visualization of enteric pathogens and minimizes the problem of delayed lactose fermentation. Due to the combination of thiosulphate with ferric ammonium citrate, hydrogen sulphide (H2S)-producing colonies form black centres.
Hoben et al (3) added Novobiocin (15 mg/litre) to improve the selectivity of the conventional medium by inhibiting Citrobacter and Proteus species. Taylor and Schelhaut (4) found the medium valuable for differentiating pathogenic organisms and for better growth of Shigellae. Inoculate the medium with fresh faeces suspended in Ringers solution or inoculate directly with rectal swabs. Spread out the inoculum to obtain isolated colonies and incubate at 35°C for 18-24 hours. Further incubation will improve differentiation between Salmonellae and Shigellae. Proteus species may resemble Salmonellae or Shigellae, hence further testing must be carried out for confirmation.
Quality Control
Appearance of Powder
Light yellow w/ tan cast coloured, homogeneous, free flowing powder.
Gelling
Firm, comparable with 1.5% Agar gel.
Colour and Clarity
Green coloured, clear to slightly opalescent gel forms in petri plates.
Reaction
Reaction of 7.67% w/v solution is pH 7.5± 0.2 at 25°C.
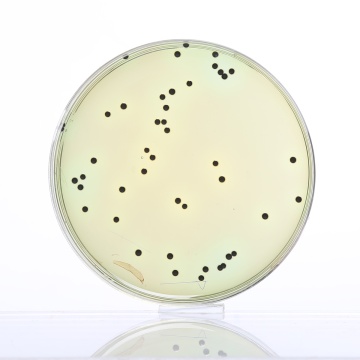
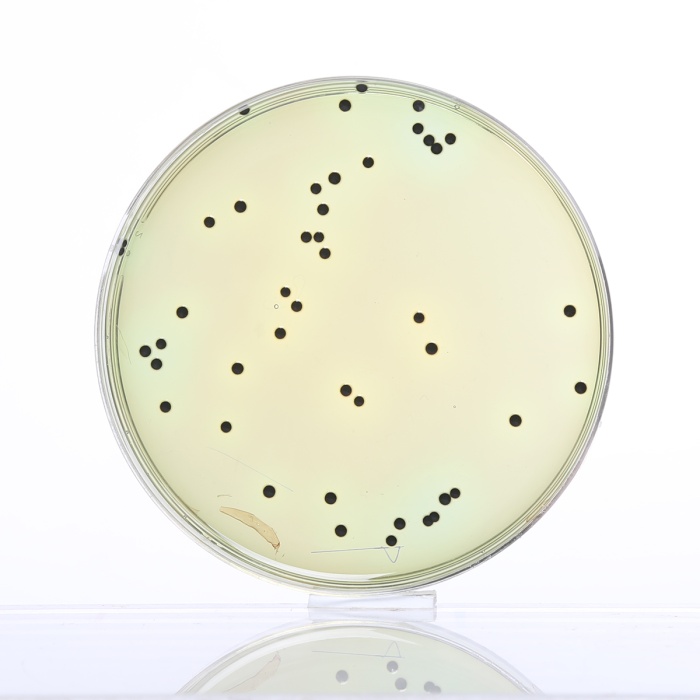
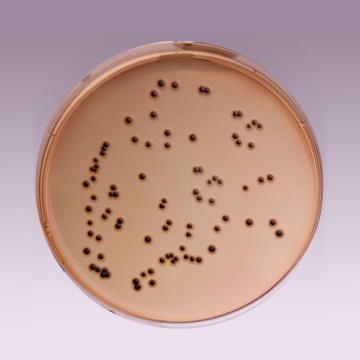
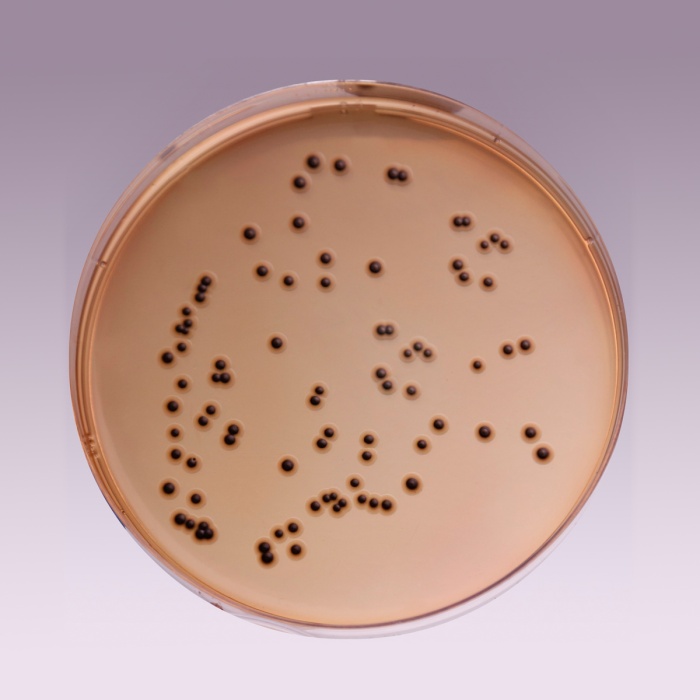
Cultural Response
Cultural characteristics observed after an incubation at 35-37°C for 18-24 hours.
| Organisms (ATCC) | Inoculum (CFU) | Growth | Recovery | Colour of Colony |
|---|---|---|---|---|
| Enterobacter aerogenes (13048) | 102 - 3x102 | fair-good | >30% | Salmon-orange |
| Enterococcus faecalis (29212) | 102 - 103 | inhibited | 0% | - |
| Escherichia coli (25922) | 102 - 3x102 | fair | >20% | Orange |
| Salmonella serotype Enteritidis (13076) |
102 - 3x102 | luxuriant | >50% | greenish blue* |
| Salmonella serotype Typhimurium (14028) |
102 - 3x102 | luxuriant | >50% | greenish blue* |
| Shigella flexneri (12022) | 102 - 3x102 | luxuriant | >50% | greenish blue |
Key: * = may have black centers (H2S production).
| Product Name | Hektoen Enteric HiVeg® Agar |
|---|---|
| SKU | MV467 |
| Product Type | HiVeg™ |
| Physical Form | Powder |
| Origin | Animal Free (Veg) |
| Packaging type | HDPE |
| References | 1.King S. and Metzger W. I., 1967, Abstr. M99, p. 7 2.King S. and Metzger W. I., 1968, Appl. Microbiol., 16:577. 3.King S. and Metzger W. I., 1968, Appl. Microbiol., 16:579. 4.MacFaddin J. F., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. I. Williams &Wilkins, Baltimore, Md. 5.Murray P. R., Baron E. J., Jorgensen J. H., Pfaller M. A., Yolken R.H., (Eds.), 8th Ed., 2003, Manual of Clinical Microbiology,ASM, Washington, D.C. 6.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 7.FDA Bacteriological Analytical Manual, 2005, 18th Ed., AOAC, Washington, DC. 8.Williams, (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed., AOAC,Washington, D.C 11.Taylor W.I. and Schelhaut, 1971, Appl. Microbiol., 21:32. 12.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition. 13.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |
| Customized Product Available | No |