Your enquiry has been submitted
Your enquiry has been submitted
Oxytetra Glucose Yeast Agar Base, Granulated (OGYE Agar Base, Granulated)
Fungi-Yeasts and Molds#CC293D
Composition**
| Ingredients | Gms / Litre |
|---|---|
| Yeast extract | 5.000 |
| Dextrose | 20.000 |
| Agar | 12.000 |
| Final pH (at 25°C) | 7.0±0.2 |
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 18.5 grams in 500 ml distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C and aseptically add reconstituted contents of one vial of Oxytetra Selective Supplement (FD032). Mix well and pour into sterile Petri plates.
Principle And Interpretation
Acidic media are not completely suitable for counting yeasts and moulds in foods since yeast cells, stressed by heat do not tolerate the acidic conditions necessary to inhibit bacterial contamination. Yeast and mould growth is often limited by the presence of acid-tolerant bacterial flora. Therefore it is evident that more active media and different selective agents are needed in order to deal with various kinds of foodstuffs, incubation conditions and types of microorganisms to be studied. Under certain conditions and when testing certain foods like milk and milk products, the use of oxytetracycline alone was not sufficient to obtain reliable yeast and mould counts.
OGYE Agar Base is formulated by Mossel et al for the selective isolation and enumeration of yeast and moulds from foods (1, 2). They found that addition of Oxytetra selective supplement to a neutral pH medium increased the recovery / count of yeast and moulds as compared to acidified medium.
Yeast extract provides essential growth nutrients. Dextrose acts as carbon and energy source. Low pH helps to reduce the bacterial flora. Oxytetracycline makes the medium more selective by inhibiting the growth of Lactobacilli encountered in milk and milk-products at low pH. The choice of a suitable media for enumeration of yeasts and moulds greatly depends on the nature of foodstuffs to be tested and the organisms that grow on them. These media remain bacteriostatic when inoculated with not greater than 1 ml of a 10-¹ food dilution and incubation at 22°C. The number of yeasts or moulds is calculated per one gram or 1 ml of sample under investigation by multiplying the number of colonies with the dilution factor. Lactic acid bacteria are inhibited on this medium.
Quality Control
Appearance
Cream to light yellow coloured granular medium
Gelling
Firm, comparable with 1.2% Agar gel.
Colour and Clarity of Prepared medium
Light amber coloured clear to slightly opalescent gel forms in Petri plates
Reaction
Reaction of 3.7% w/v aqueous solution at 25°C. pH : 7.0±0.2
pH
6.80-7.20
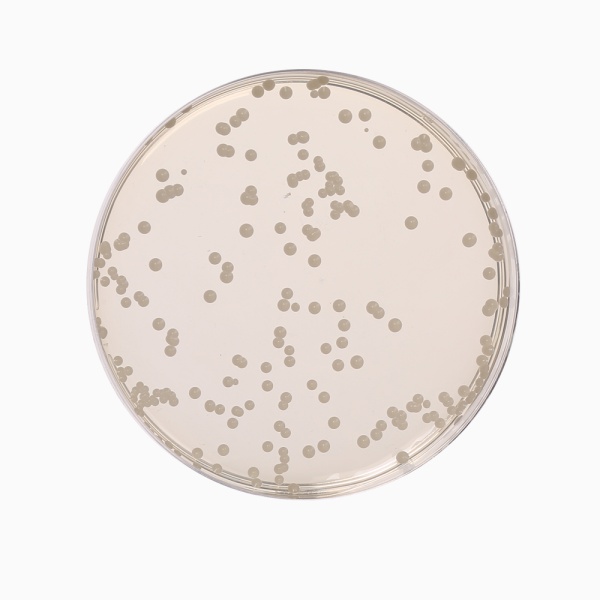
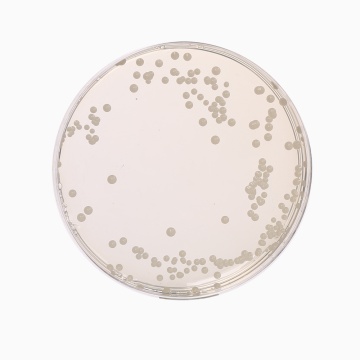
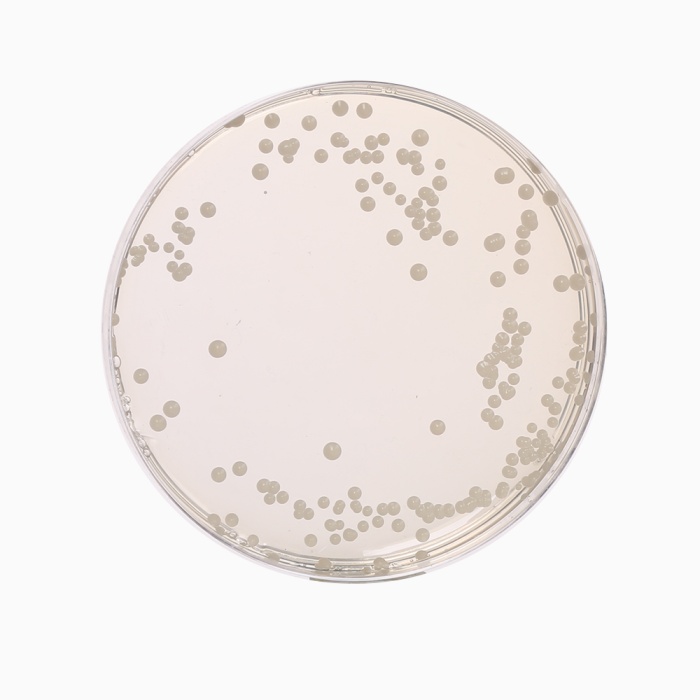
Cultural Response
Cultural characteristics observed with added 1 vial of Oxytetra Selective Supplement (FD032), after an incubation at 25-30°C after 2-5 days.
| Organism | Inoculum (CFU) | Growth | Recovery |
|---|---|---|---|
| *Aspergillus brasiliensis ATCC 16404 | 50-100 | good-luxuriant | |
| Candida albicans ATCC 10231 | 50-100 | good-luxuriant | >=50% |
| Escherichia coli ATCC 25922 | >=10³ | inhibited | 0% |
| Saccharomyces cerevisiae ATCC 9763 | 50-100 | good-luxuriant | >=50% |
| Saccharomyces uvarum ATCC 9080 | 50-100 | good-luxuriant | >=50% |
*Key: Formerly known as Aspergillus niger ATCC 16404
Storage and Shelf Life
Store below 30°C in tightly closed container and the prepared medium at 2 - 8°C. Use before expiry date on the label.
| Product Name | Oxytetra Glucose Yeast Agar Base, Granulated (OGYE Agar Base, Granulated) |
|---|---|
| SKU | GM639 |
| Product Type | Granulated |
| Physical Form | Granular |
| Origin | Animal Free (Microbial) |
| Packaging type | HDPE |
| References | 1.Mossel D. A. A., Kleynen-Semmeling H. M., Vincentie H., Beerens H. and Catsaras M., 1970, J. Appl. Bacteriol., 33:4542.Mossel D. A. A., Visser M. and Mengerink W. H. J., 1962, Lab. Pract. 11:109. 3.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 4.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition. 5.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |
| Customized Product Available | No |