Your enquiry has been submitted
Your enquiry has been submitted
Columbia Blood HiCynth™ Agar Base w/1% Agar
Intended Use
Recommended a basal medium used with or without blood for isolation and cultivation of fastidious bacteria from various samples.
Composition
| Ingredients | Gms/Litre |
|---|---|
| HiCynth™ Peptone No.3* | 23.000 |
| Corn starch | 1.000 |
| Sodium chloride | 5.000 |
| Agar | 10.000 |
| Final pH (at 25°C) | 7.3±0.2 |
**Formula adjusted, standardized to suit performance parameters
*Chemically defined peptone
Directions
Suspend 39 grams in 1000 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C before adding heat sensitive compounds.
For Blood Agar: Add 5% v/v sterile defibrinated sheep blood to sterile cool base.
For Chocolate Agar: Add 10% v/v sterile defibrinated sheep blood to sterile cool base. Heat to 80°C for 10 minutes with constant agitation. The medium can be made selective by adding different antimicrobials to sterile base.
For Brucella species: Add rehydrated contents of 1 vial of NPBCVN Selective Supplement (FD005) to 500 ml sterile molten base.
For Campylobacter species: Add rehydrated contents of 1 vial of Blaser-Wang Selective Supplement (FD006) or Butzler Selective Supplement (FD007) or Skirrow Selective Supplement (FD008) or VTCA Selective Supplement (FD090) or Butzler VI Selective Supplement (FD106) to 500 ml sterile molten base along with rehydrated contents of 1 vial of Minerals Growth Supplement (FD009).
For Gardnerella species: Add rehydrated contents of 1 vial of GNA Selective Supplement (FD056) to 500 ml sterile molten base.
For Cocci: Add rehydrated contents of 1 vial of NC Selective Supplement (FD030) or NNP Selective Supplement (FD031) or CO Selective Supplement (FD119) to 500 ml sterile molten base.
Principle And Interpretation
Columbia Blood Agar Base w/1% Agar is a general-purpose nutritious agar base formulated by Ellner et al (1) in 1966; it is further enriched by the addition of sterile blood. Traditionally blood agar bases have incorporated either casein hydrolysate to give rapid production of large colonies or meat infusion to give defined hemolytic reactions. The media combines both to give an improved all round performance.
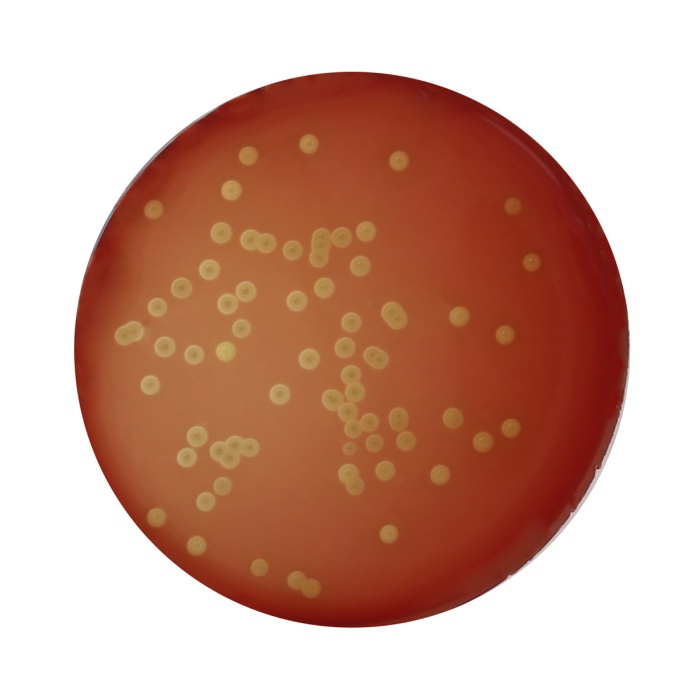
Columbia Blood HiCynth™ Agar Base w/1% Agar is the modification of the same, but prepared by replacing animal and vegetable peptones with chemically defined peptones to avoid BSE/TSE risks associated with animal peptones. This medium with the added HiCynth™ Peptone No.3 supports rapid and luxuriant growth of fastidious and nonfastidious organisms. Also, this medium promotes typical colonial morphology; better pigment production and more sharply defined haemolytic reactions. Corn starch serves as an energy source and also neutralizes toxic metabolites. Sheep blood permits the detection of haemolysis and also provides heme (X factor) which is required for the growth of many bacteria. However it is devoid of V factor (Nicotinamide adenine dinucleotide) and hence Haemophilus influenzae which needs both the X and V factors, will not grow on this medium. As this medium has a relatively high carbohydrate content, beta-haemolytic Streptococci may exhibit a greenish haemolytic reaction which may be mistaken for the alpha haemolysis. Carry out confirmatory tests of all the colonies.
Columbia Blood HiCynth™ Agar Base w/1% Agar is used as the base for the media containing blood and for selective media formulations in which different combinations of antimicrobial agents are used as additives. HiCynth™ Agar Base w/1% Agar with added sterile serum provides an efficient medium for Corynebacterium diphtheriae virulence test medium.
Specific Cultivation Guidelines
After following the established technique for C. diphtheriae, lines of toxin-antitoxin precipitation are clearly visible in 48 hours. Many pathogens require carbon dioxide; therefore, plates may be incubated in an atmosphere containing approximately 3-10% CO2.
Precaution: Brucella cultures are highly infective and must be handled carefully; incubate in 5-10% CO2. Campylobacter species are best grown at 42°C in a microaerophillic atmosphere. Plates with Gardenerella supplements should be incubated at 35°C for 48 hours containing 7% CO2 (2).
Type of specimen
Food samples
Specimen Collection and Handling
For food samples, follow appropriate techniques for sample collection and processing as per guidelines (3). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Individual organisms differ in their growth requirement and may show variable growth patterns on the medium.
- Further biochemical tests must be carried out for confirmation.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance
Cream to yellow homogeneous free flowing powder
Gelling
Firm, comparable with 1.0% Agar gel.
Colour and Clarity of prepared medium
Basal medium :Light amber coloured clear to slightly opalescent gel. After addition of 5%v/v sterile defirinated blood : Cherry red coloured opaque gel forms in Petri plates
Reaction
Reaction of 3.9% w/v aqueous solution at 25°C. pH : 7.3±0.2
pH
7.10-7.50
| Organism | Inoculum (CFU) | Growth | Recovery | Haemolysis |
|---|---|---|---|---|
| Neisseria meningitidis ATCC 13090 | 50-100 | luxuriant | >=70% | none |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | 50-100 | luxuriant | >=70% | beta / gamma |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | 50-100 | luxuriant | >=70% | beta/gamma |
| Staphylococcus epidermidis ATCC 12228 (00036*) | 50-100 | luxuriant | >=70% | gamma |
| Streptococcus pneumoniae ATCC 6303 | 50-100 | luxuriant | >=70% | alpha |
| Streptococcus pyogenes ATCC 19615 | 50-100 | luxuriant | >=70% | beta |
Key: (*) Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (4,5).
| Product Name | Columbia Blood HiCynth™ Agar Base w/1% Agar |
|---|---|
| SKU | MCD144A |
| Product Type | HiCynth™ |
| Physical Form | Powder |
| Origin | Chemically defined (HiCynth™) |
| Packaging type | HDPE |
| References | 1. Ellner P. P., Stoessel C. J., Drakeford E. and Vasi F., 1966, Am. J. Clin. Pathol., 45:502. 2.Fildes P., 1920, Br. J. Exp. Pathol., 1:129. 3.Fildes P., 1921, Br. J. Exp. Pathol., 2:16. 4.Chapin K. C. and Doern G. V., 1983, J. Clin. Microbiol., 17:1163. 5.Bailey R. K., Voss J. L. and Smith R. F., 1979, J. Clin. Microbiol., 9 ; 65-71 6.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 7.Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water andWastewater, 23rd ed., APHA, Washington, D.C. 8.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 10.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manualof Clinical Microbiology, 11th Edition. Vol. 1. |