Your enquiry has been submitted
Your enquiry has been submitted
Middlebrook 7H9 Broth Base
Non Egg Media#CC293D
Intended Use
Recommended for cultivation and sensitivity testing of Mycobacterium tuberculosis.
Composition**
| Ingredients | Gms / Litre |
|---|---|
| Ammonium sulphate | 0.500 |
| Disodium hydrogen phosphate | 2.500 |
| Potassium dihydrogen phosphate | 1.000 |
| Sodium citrate | 0.100 |
| Magnesium sulphate | 0.050 |
| Calcium chloride anhydrous | 0.0005 |
| Zinc sulphate | 0.001 |
| Copper sulphate | 0.001 |
| Ferric ammonium citrate | 0.040 |
| L-Glutamic acid | 0.500 |
| Pyridoxine hydrochloride | 0.001 |
| Biotin | 0.0005 |
Final pH ( at 25°C): 6.6±0.2
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 2.35 grams in 450 ml purified/distilled water. Add either 2 ml glycerol or 0.5 g polysorbate 80. Heat if necessary to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 10 minutes. Cool to 45-50°C and aseptically add contents of 1 vial of Middlebrook ADC Growth Supplement (FD019). Mix well before dispensing in tubes or flasks as desired.
Principle And Interpretation
Media for Mycobacterial cultivation may be egg-based (Lowenstein Jensen Media) or agar-based (Middlebrook Media) (1). Dubos and Middlebrook (2) developed various formulations containing oleic acid and albumin, which protect Mycobacterium from toxic agents, helping for the growth of tubercle bacilli. Middlebrook 7H9 Broth Base was formulated by Middlebrook (3) and Middlebrook et al and Schaeffer (4,5). This medium with Middlebrook ADC Growth Supplement (FD019) and glycerol or polysorbate 80 is also recommended for cultivation of Mycobacteria and for assaying the INH content of the patients sera. The medium can also be used for preparing inocula for antimicrobial assays, as a basal medium for biochemical tests and for the subculture of stock strains.
Middlebrook media consists of many inorganic salts, which help, in growth of Mycobacteria. Citric acid formed from sodium citrate helps in retaining inorganic cations in solution. Glycerol supplies carbon and energy. Long chain fatty acids are essential for metabolism of Mycobacteria. Middlebrook ADC Growth Supplement (FD019) contains bovine albumin, dextrose and catalase. Some free fatty acids are toxic to Mycobacteria but albumin binds to those fatty acids and prevents toxic action on Mycobacteria. Dextrose serves as an energy source. Catalase neutralizes toxic peroxides. Mycobacteria grow more rapidly in broth media; therefore primary isolation of all specimens can be done in Middlebrook 7H9 Broth Base. After processing the sample as required, inoculate the media with the test specimen.
Mycobacteria are strict aerobes and therefore increased CO2 tension and aerobic conditions must be provided during incubation. Care should be taken while decontamination of the specimen. Also proper specimen collection (sputum and not saliva) should be ensured. Samples should be carefully handled to avoid contamination.
Type of specimen
Clinical samples : Sputum
Specimen Collection and Handling
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (6,7). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
In Vitro diagnostic use only. For professional use only. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Mycobacteria are strict aerobes and therefore increased CO2 tension and aerobic conditions must be provided during incubation.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Cream to beige homogeneous free flowing powder
Colour and Clarity of prepared medium: Light amber coloured clear solution in tubes
Reaction: Reaction of 0.47% w/v aqueous solution (containing either Glycerol or Polysorbate 80) at 25°C. pH : 6.6±0.2
pH: 6.40-6.80
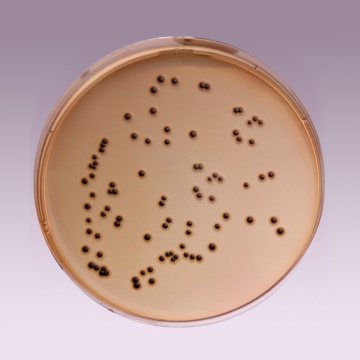
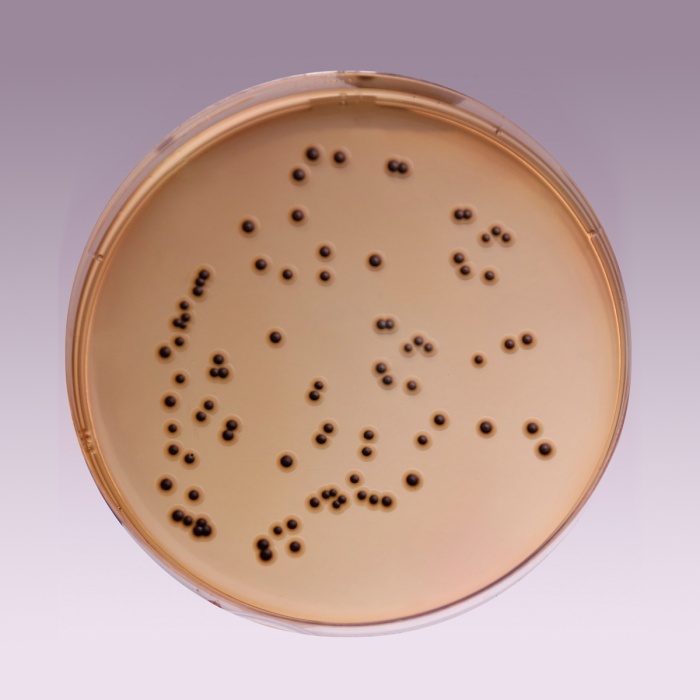
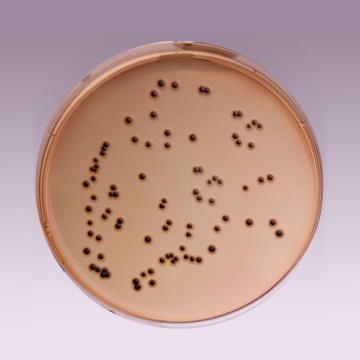
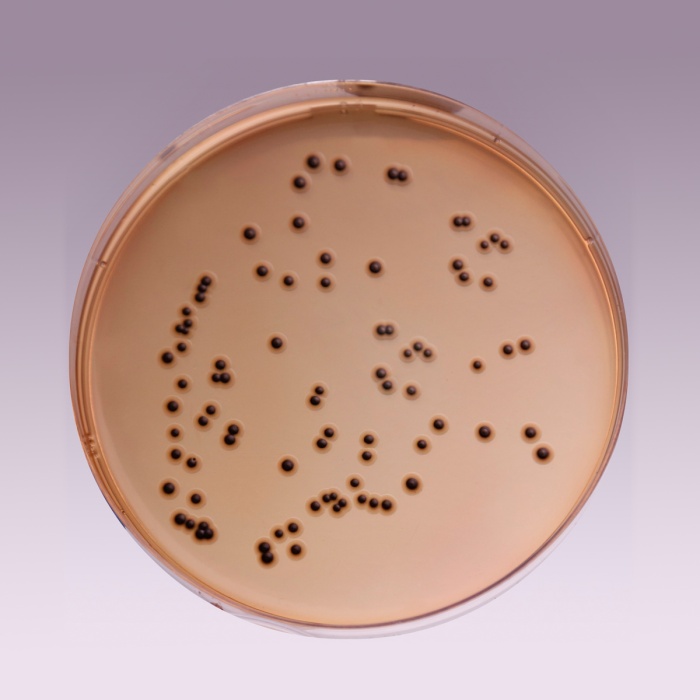
Cultural Response: Cultural characteristics observed with added Middlebrook ADC Growth Supplement (FD019) with added glycerol or Polsorbate 80 after an incubation at 35-37°C for 2-4 weeks
| Organism | Growth |
|---|---|
| Mycobacterium fortuitum ATCC 6841 | good-luxuriant |
| Mycobacterium smegmatis ATCC 14468 | good-luxuriant |
| Mycobacterium tuberculosis H37RV (25618) | good-luxuriant |
Storage and Shelf Life
Store below 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle inorder to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (6,7).
Reference
- Murray P. R., Baron J. H., Pfaller M. A., Jorgensen J. H. and Yolken R. H., (Ed.), 2003, Manual of Clinical Microbiology,8th Ed., American Society for Microbiology, Washington, D.C.
- Dubos R. J. and Middlebrook G., 1947, Am. Rev. Tuberc., 56:334.
- Middlebrook G., Fitzsimmons Army Hospital, Denver, Co, Report 1, 1955
- Middlebrook G. and Cohn M. L., 1958, Am. J. Public Health, 48:844.
- Middlebrook G., Cohn, M. L. and Schaeffer W. B.,1954, Am. Rev. Tuber, 70, 852
- Isenberg, (Ed.), Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | Middlebrook 7H9 Broth Base |
|---|---|
| SKU | M198 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1.Lennette and others (Eds.), 1985, Manual of Clinical Microbiology, 4th ed., ASM, Washington, D.C. 2.Downes F. P. and Ito K., (Eds.), 2001, Compendium of Methods for the Microbiological Examination of Foods, 4th Ed.,APHA, Washington, D.C. 3.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 4.Eaton A. D., Clesceri L. S., Rice E. W., and Greenberg A. W., (Eds.), 2005, Standard Methods for the Examination of Waterand Wastewater, 21st Ed., APHA, Washington, D.C. 5.Williams S., (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed., AOAC,Washington, D.C. 6.The United States Pharmacopoeia, 2006, USP29/NF24, The United States Pharmacopoeial Convention. Rockville, MD. 7.MacFaddin J., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. I, Williams andWilkins, Baltimore. |
| Customized Product Available | No |