The store will not work correctly in the case when cookies are disabled.
-
Home
-
Bacterial Endotoxin Assay Kit

Harmonized Gel-Clot Limit Method for Testing of Bacterial Endotoxin as per USP, EP, JP and ICH Guidelines
POTENTIAL USER SEGMENTS :
- Pharmaceuticals
- Vaccines
- Biologicals
- Hospitals
- Medical Device Manufacturers
ADVANTAGES :
- Single-test format available for all lysate sensitivities
- Ampoule packing reduces need for pyrogen free tubes as well as reconstituting lysate.
- Simply add your sample, mix and incubate!
- Highly stable Control Standard Endotoxin (CSE) provided with the kit
- Cost effective premium quality reagents compared to those of competitors
INTENDED USE :
- Qualitative detection of endotoxin
- Pyrogen control or endotoxin detection during production processes
PRINCIPLE :
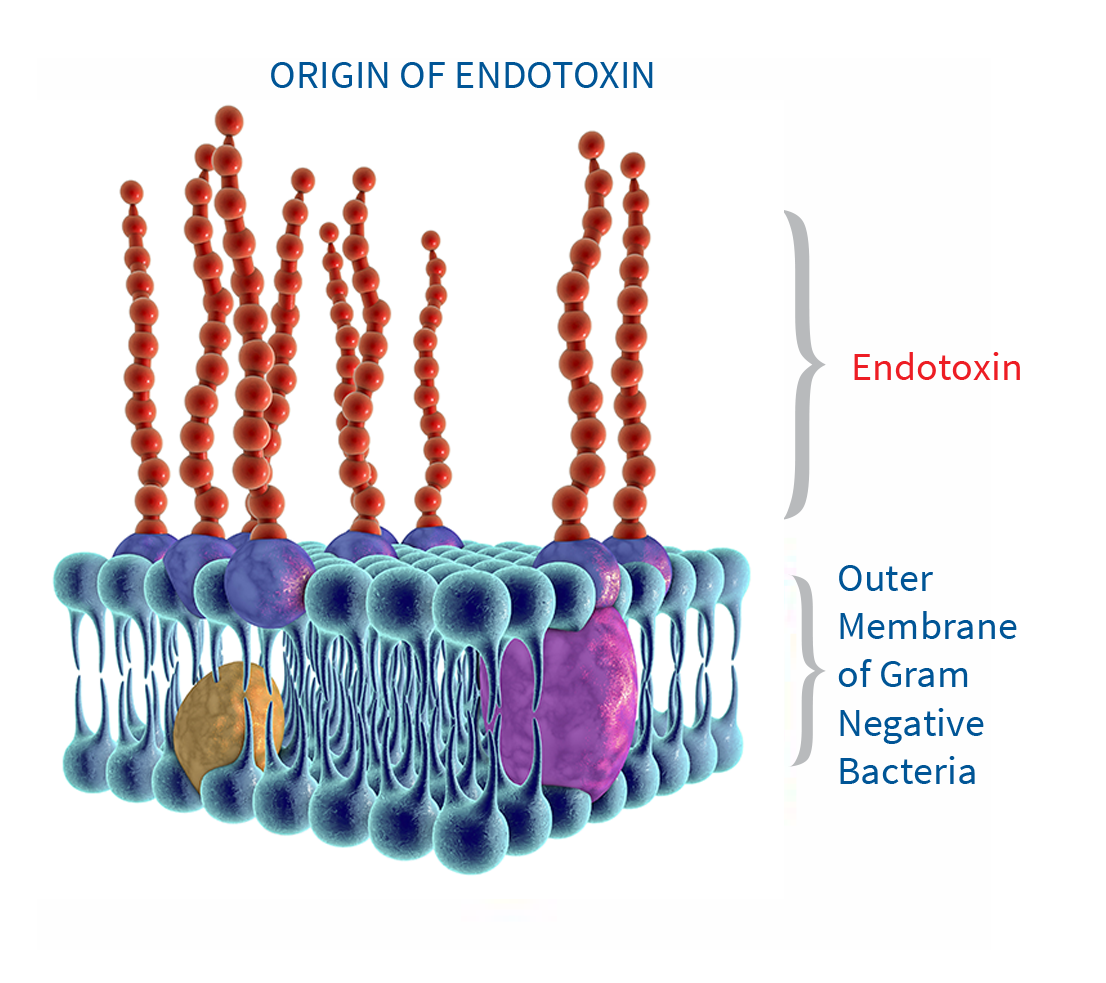
- Presence of the endotoxin activates factor C in the horseshoe crab amoebocyte lysate
- Activated factor C further activates factor B
- Pre-clotting enzyme (inactive) is cleaved by activated factor B into clotting enzyme (active)
- Active enzyme reduces Coagulogen into Coagulin thereby leading to clot formation
Download Catalogue