Your enquiry has been submitted
Your enquiry has been submitted
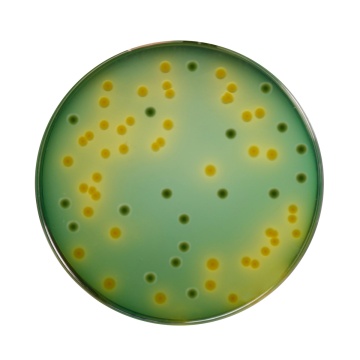
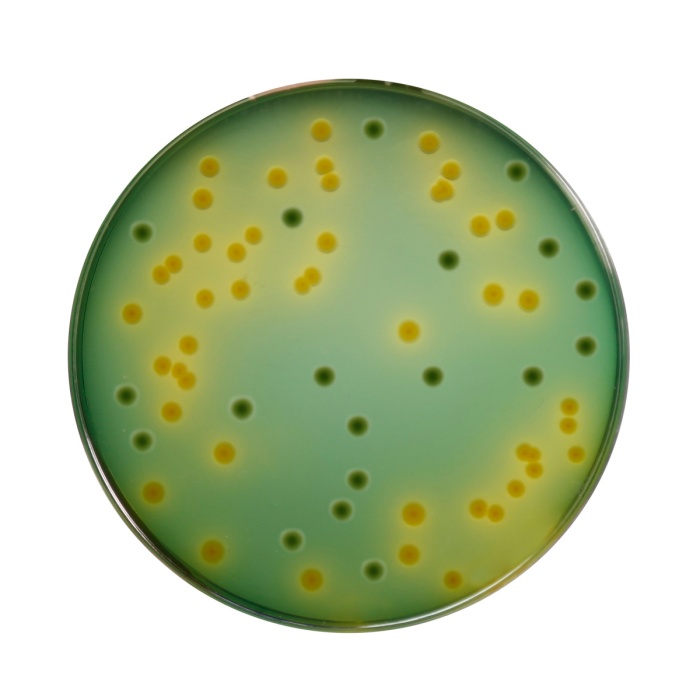
MacConkey Sorbitol HiVeg® Agar (Sorbitol HiVeg® Agar)
Intended Use
Recommended for isolation and identification of enteropathogenic Escherichia coli strains associated with infant diarrhea.
Composition
| Ingredients | g/L |
|---|---|
| HiVeg®™ peptone | 17.000 |
| HiVeg®™ peptone No. 3 | 3.000 |
| D-Sorbitol | 10.000 |
| Synthetic detergent | 1.500 |
| Sodium chloride | 5.000 |
| Neutral red | 0.030 |
| Crystal violet | 0.001 |
| Agar | 13.500 |
| Final pH (at 25°C) | 7.1±0.2 |
Formula adjusted, standardized to suit performance parameters
Directions
Suspend 50.03 grams in 1000 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. AVOID OVERHEATING. Cool to 45-50°C and pour into sterile Petri plates.
Principle And Interpretation
MacConkey Sorbitol Agar is based on the formulation described by Rappaport and Henigh (1). This medium is recommended for isolation of enteropathogenic Escherichia coli O157: H7, which ferments lactose but does not ferment sorbitol, hence produces colourless colonies. This organism has been recognized as a cause of hemorrhagic colitis (2). E.coli O157: H7 is a human pathogen associated with hemorrhagic colitis that results from the action of a shiga-like toxin (SLT) (3,4). On standard MacConkey Agar containing lactose, this strain is indistinguishable from other lactose-fermenting E.coli. In MacConkey Sorbitol Agar Base, lactose is replaced by sorbitol. Unlike most E.coli strains, E.coli O157:H7 ferments sorbitol slowly or not at all (5,6). The growth of E.coli O157:H7 on MacConkey Agar with Sorbitol shows colourless colonies and most of the fecal flora ferment sorbitol and appear pink. MacConkey Agar with Sorbitol therefore permits ready recognition of E.coli O157:H7 (3,4,7). Sorbitol HiVeg® Agar is prepared by using vegetable peptones in place of animal based peptones which make the media free of BSE/TSE risks. HiVeg®™ peptone and HiVeg®™ peptone No. 3 supply necessary nutrients like nitrogenous and carbonaceous compounds, long chain amino acids, minerals, vitamins and trace ingredients for the growth of organisms. Crystal violet and Synthetic detergent present in the medium inhibit growth of gram-positive bacteria. Sodium chloride maintains osmotic equilibrium. Neutral red is an indicator. D-Sorbitol is the fermentable carbohydrate.
Type of specimen
Food and Dairy samples.
Specimen Collection and Handling
For food and dairy samples, follow appropriate techniques for sample collection and processing as per guidelines (5,8,9). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- MacConkey Sorbitol Agar however should not be solely used to detect pathogenic E.coli O157: H7 strains as some non-toxic strains will also not ferment sorbitol (4).
- Further biochemical tests must be carried out for further confirmation.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance
Light yellow to pink homogeneous free flowing powder
Gelling
Firm, comparable with 1.35% Agar gel.
Colour and Clarity of prepared medium
Purplish red coloured clear to slightly opalescent gel forms in Petri plates
Reaction
Reaction of 5.0% w/v aqueous solution at 25°C. pH: 7.1±0.2
pH
6.90-7.30
Cultural Response
Cultural characteristics observed after an incubation at 35-37°C for 18-24 hours.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| Salmonella Typhi ATCC 6539 | 50-100 | luxuriant | >=50% | pink |
| Shigella flexneri ATCC 12022 (00126*) | 50-100 | luxuriant | >=50% | colourless |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | luxuriant | >=50% | pink |
| Escherichia coli serotype O11 and O55 | 50-100 | luxuriant | >=50% | colourless |
| Escherichia coli O157:H7 NCTC 29900 | 50-100 | luxuriant | >=50% | colourless |
Key: *Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (5,8).
| Product Name | MacConkey Sorbitol HiVeg® Agar (Sorbitol HiVeg® Agar) |
|---|---|
| SKU | MV298 |
| Product Type | HiVeg™ |
| Physical Form | Powder |
| Origin | Animal Free (Veg) |
| Packaging type | HDPE |
| References | 1.Rappaport F. and Henigh E., 1952, J. Clin. Pathol., 6 : 361. 2.Karmali M. A., Petric M., Lim C. et al, 1985, J. Infect. Dis.,151:775. 3.Sanderson M. W., Gay J. M., Hancock D. D., Gay C. C., Fox L. K. and Besser T. E., 1955, J. Clin. Microbiol., 33: 2616. 4.Pelczar M. J., Chan E. C. and Kreig M. R., 1986, Microbiology, 5th Ed., McGraw Hill Book Co., New York,5.March S. B. and Ratnam S., 1986, J. Clin. Microbiol., 23:869. 6.Centre for Diseases Control, 1991, Morbid. Mortal, Weekly Rep 40:265. 7.Murray P. R., Baron J. H., Pfaller M. A., Tenover F. C. and Yolken R. H. (Ed.), 1999, Manual of Clinical Microbiology, 7thEd. American Society for Microbiology, Washington, D. C. 8.Zadik J. M., Chapman P. A. and Siddons C. A., 1993, J. Med. Microbiol., 39:155. 9.American Public Health Association, Standard Methods for the Examination of Dairy Products, 1978, 14th Ed., WashingtonD.C.10.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 11.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition. 12.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1.1 3.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. |
| Customized Product Available | No |