Your enquiry has been submitted
Your enquiry has been submitted
TCBS Agar
Intended Use
Recommended for the selective isolation and cultivation of Vibrio cholerae and other enteropathogenic Vibrio's causing food poisoning from clinical and food specimen.
Composition**
| Ingredients | g / L |
|---|---|
| Proteose peptone | 10.000 |
| Yeast extract | 5.000 |
| Sodium thiosulphate | 10.000 |
| Sodium citrate | 10.000 |
| Bile | 8.000 |
| Sucrose | 20.000 |
| Sodium chloride | 10.000 |
| Ferric citrate | 1.000 |
| Bromo thymol blue | 0.040 |
| Thymol blue | 0.040 |
| Agar | 15.000 |
Final pH ( at 25°C): 8.6±0.2
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 89.08 grams in 1000 ml purified/ distilled water. Heat to boiling to dissolve the medium completely. DO NOT AUTOCLAVE. Cool to 45-50°C. Mix well and pour into sterile Petri plates.
Principle And Interpretation
TCBS Agar was developed by Kobayashi et al (1), who modified the selective medium of Nakanishi (2). Although this medium was originally designed for the isolation of V.cholerae and V. parahaemolyticus, most Vibrio's grow to healthy large colonies with many different colonial morphologies. TCBS Agar is also recommended by APHA for the selective isolation of V. cholerae and V. parahaemolyticus (3,4). Enrichment in Alkaline Peptone Water (M618), followed by isolation on TCBS Agar is routinely used for isolation of V.cholerae (5,6,7).
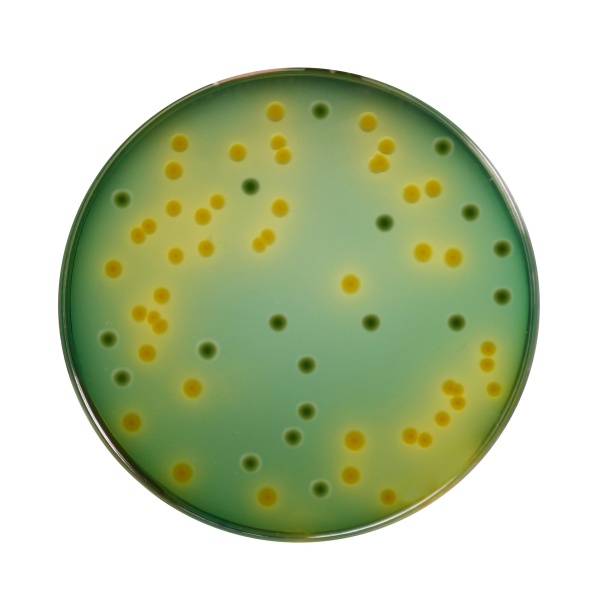
Proteose peptone and yeast extract provide nitrogenous compounds, vitamin B complex and other essential growth nutrients. Bile, a derivative of bile salts and sodium citrate inhibit gram-positive bacteria and coliforms (8). Sodium thiosulphate serves as a good source of sulphur, which in combination with ferric citrate detects the production of hydrogen sulphide. For the metabolism of Vibrio's, sucrose is added as a fermentable carbohydrate. Vibrio that is able to utilize sucrose will from yellow colonies. Bromothymol blue and thymol blue are the pH indicators. The alkaline pH of the medium improves the recovery of V.cholerae. Strains of V. cholerae produce yellow colonies on TCBS Agar because of fermentation of sucrose. V. alginolyticus also produce yellow colonies. V.parahaemolyticus is a sucrose non-fermenting organism and therefore produces blue-green colonies, as does V.vulnificus.
Type of specimen
Clinical : faeces, etc; Food samples; Water samples.
Specimen Collection and Handling
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (7,8). For food and dairy samples, follow appropriate techniques for sample collection and processing as per guidelines (4,9,10). For water samples, follow appropriate techniques for sample collection, processing as per guidelines and local standards (3). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
In Vitro diagnostic use. For professional use only. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- The medium should be inoculated heavily with faecal specimens because growth of few species may be inhibited on the medium due to fermentation of sucrose and accumulation of acids.
- However, occasional isolates of Pseudomonas and Aeromonas may also form blue green colonies on TCBS Agar (11).
- Proteus species that are sucrose-fermenters may form yellow colonies (11).
- TCBS Agar is not a suitable medium for oxidase testing of Vibrio species (12).
- A few strains of V. cholerae may appear green or colourless on TCBS Agar due to delayed sucrose fermentation (11).
- TCBS Agar is highly selective for Vibrio species. Any H2S negative colony of TCBS Agar can be considered presumptive positive for Vibrio.
- Further biochemical and serological tests must be carried out for complete identification.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance Light yellow to light tan homogeneous free flowing powder
Gelling Firm, comparable with 1.5% Agar gel
Colour and Clarity of prepared medium Bluish green coloured clear to slightly opalescent gel forms in Petri plates.
Reaction Reaction of 8.9% w/v aqueous solution at 25°C. pH : 8.6±0.2
pH 8.40-8.80
Cultural Response
Cultural characteristics observed after an incubation at 35-37°C for 18-24 hours.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| Productivity | ||||
| Vibrio parahaemolyticus NCTC 10885 (00185*) | 50-100 | good-luxuriant | >=50% | blue |
| Vibrio furnissii NCTC 11218 (00186*) | 50-100 | good-luxuriant | >=50% | greenish yellow |
| Specificity | ||||
| Escherichia coli ATCC 25922 (00013*) | >=104 | inhibited | 0% | |
| Escherichia coli ATCC 8739 (00012*) | >=104 | inhibited | 0% | |
| Escherichia coli ATCC 11775 (00090*) | >=104 | inhibited | 0% | |
| Additional Microbiological Testing | ||||
| Vibrio cholerae ATCC 15748 | 50-100 | good-luxuriant | >=50% | yellow |
| Vibrio parahaemolyticus ATCC 17802 (00037*) | 50-100 | good-luxuriant | >=50% | blue |
| Vibrio fluvialis ATCC 33809 (00137*) | 50-100 | good-luxuriant | >=50% | yellow |
| Vibrio vulnificus ATCC 29306 | 50-100 | fair - good | >=20% | greenish yellow |
| ## Proteus hauseri ATCC 13315 | >=104 | inhibited | 0% | |
| Shigella flexneri ATCC 12022 (00126*) | >=104 | inhibited | 0% | |
| Enterococcus faecalis ATCC 29212 (00087*) | >=104 | inhibited | 0% | |
Key: (*) Corresponding WDCM numbers ## Formerly known a Proteus vulgaris
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (7,8).
Reference
- Kobayashi T., Enomoto S., Sakazaki R., and Kuwahara S., 1963, Jap. J. Bacteriol., 18: 387.
- Nakanishi Y., 1963, Modern Media 9: 246.
- Lipps WC, Braun-Howland EB, Baxter TE,eds. Standard methods for the Examination of Water and Wastewater, 24th ed. Washington DC:APHA Press; 2023.
- Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Forbes B. A., Sahm A. S. and Weissfeld D. F., 1998, Bailey & Scotts Diagnostic Microbiology, 10th Ed., Mosby, Inc. St. Louis, Mo.
- Furniss A. L., Lee J. V. and Donovan T. J., 1978, The Vibrios, Public Health Laboratory Service Monograph Series No. 11, Maidstone Public Health Laboratory, H.M.S.O., London, England.
- Murray P. R., Baron J. H., Pfaller M. A., Jorgensen J. H. and Yolken R. H., (Eds.), 2003, Manual of Clinical Microbiology, 8th Ed., American Society for Microbiology, Washington, D.C.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.
- Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C.
- American Public Health Association, Standard Methods for the Examination of Dairy Products, 1978, 14th Ed., Washington D.C.
- MacFaddin J. F., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. 1, Williams & Wilkins, Baltimore, Md.
- Morris G. K., Merson M. H., Huq A. K., Kibrya A. K. and Black R., 1979, J. Clin. Microbiol., 9:79
| Product Name | TCBS Agar |
|---|---|
| SKU | M189 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Group A Streptococcal disease,Centers for Disease Control and Prevention. Retrieved November 21, 2012. 2.Murray,P.R,E.J.Baron,M.A.Pfaller,F.C.Tenover, and R.H. Yolken(eds.).Manual of clinical microbiology,6th ed.AmericanSociety of Microbiology,Washington,D.C. |
| Customized Product Available | No |