Your enquiry has been submitted
Your enquiry has been submitted
Tryptone Bile Agar
Intended Use
Recommended for rapid detection and enumeration of Escherichia coli in food using a modified direct plating method.
Composition
| Ingredients | Gms/Litre |
|---|---|
| Tryptone | 20.000 |
| Bile salts mixture | 1.500 |
| Agar | 15.000 |
| Final pH (at 25°C) | 7.2±0.2 |
Formula adjusted, standardized to suit performance parameters
Directions
Suspend 36.5 grams in 1000 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C. Mix well and pour into sterile Petri plates.
Principle And Interpretation
Tryptone Bile Agar was formulated by Anderson and Baird-Parker (1). The International Commission on the Microbiological Specifications for Foods (CMSF) (6) compared the Most Probable Number (MPN) and the Anderson-Baird-Parker Direct Plating Method (DPM) and observed that DPM was superior to MPN for enumeration of Escherichia coli from raw meats. Superiority of DPM method was noticed by the organization on the basis of less variability, better recovery from frozen samples, greater rapidity and the smaller quantity of medium required. The DPM enumerates both anaerogenic and late lactose fermenting strains of E. coli which could be missed by the MPN method (about 10%)(3). This formulation is recommended by ISO committee for the enumeration of E. coli (7). Holbrook et al (5) modified the DPM for detection and enumeration of sublethally damaged cells of E. coli in frozen, dried, heat processed or acid foods and found that resuscitation step reduces the high concentration of sugar present in the inoculum to a level which does not interfere with the production of indole as the synthesis of tryptophanase is inhibited by high sugar concentrations (2). Certain organisms breakdown the amino acid tryptophan with the help of enzymes that mediate the production of indole by hydrolytic activity (10). The indole produced can be detected by either Kovacs or Ehrlichs reagent (4). Indole combines with the aldehyde present in the above reagent to give red colour in the alcohol layer. The alcohol layer extracts and concentrates the red colour complex. The indole positive organisms other than E. coli are inhibited by bile salts and elevated incubation temperature.
Type of specimen
Food samples: meat and meat product
Specimen Collection and Handling
For food samples, follow appropriate techniques for sample collection and processing as per guidelines (11). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Due to variable nutritional requirements, some strains show poor growth on this medium.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Cream to yellow homogeneous free flowing powder
Gelling: Firm, comparable with 1.5% Agar gel
Colour and Clarity of prepared medium: Yellow coloured clear to slightly opalescent gel forms in Petri plates.
Reaction: Reaction of 3.65% w/v aqueous solution at 25°C. pH: 7.2±0.2
pH: 7.00-7.40
Cultural Response
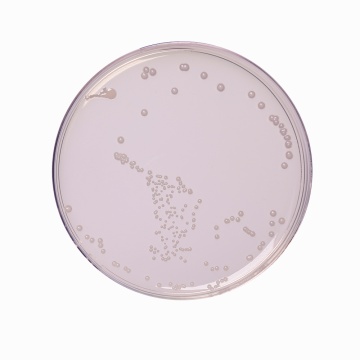
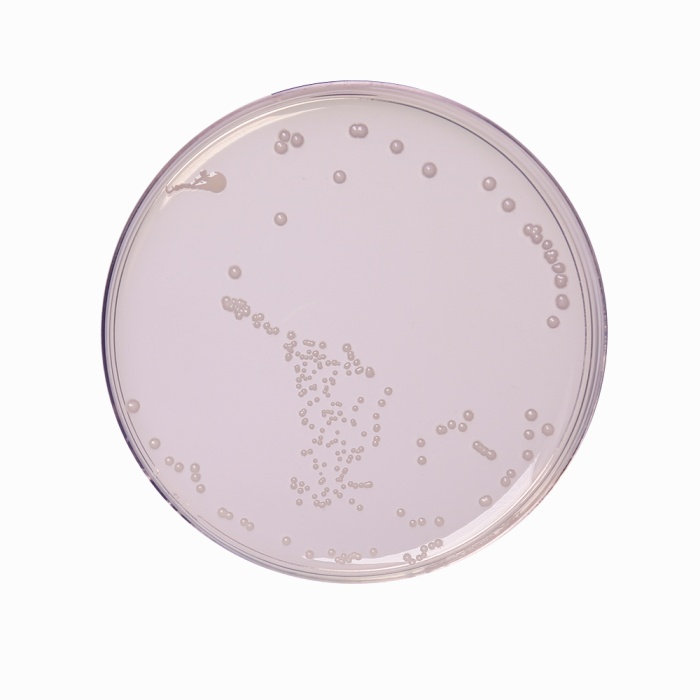
Cultural characteristics observed after an incubation at 44°C for 24 hours.
| Organism | Inoculum (CFU) | Growth | Recovery |
|---|---|---|---|
| # Klebsiella aerogenes ATCC 13048 (00175*) | >=104 | inhibited | 0% |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | good-luxuriant | >=50% |
Key: *Corresponding WDCM numbers. (#) Formerly known as Enterobacter aerogenes
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle inorder to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (8,9).
References
- Anderson J. M. and Baird-Parker A. C., 1975, J. Appl. Bacteriol., 39:111.
- Clarke P. H. and Cowen S. T., 1952, J. Gen. Microbiol., 6:187.
- Ewing W. H., 1972, US Dept. of Health, Education and Welfare, CRC, Atlanta.
- Finegold S. M., Baron E. J., 1986, Bailey and Scotts Diagnostic Microbiology, 7th Ed., The C.V. Mosby Co., St. Louis.
- Holbrook R., Anderson J. M. and Baird - Parker A.C., 1980, Food Technol. in Aust., 32:78.
- International Commission on Microbiological Specifications for Food, 1979, Can. J. Microbiol., 25:1321.
- International Organization for Standardization (ISO), 1988, Draft ISO/DIS 6391.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
- MacFaddin J. F., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. I, Williams and Wilkins, Baltimore.
- Salfinger Y., and Tortorello M.L., 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
| Product Name | Tryptone Bile Agar |
|---|---|
| SKU | M961 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Anderson J. M. and Baird-Parker A. C., 1975, J. Appl. Bacteriol., 39:1 |