Your enquiry has been submitted
Your enquiry has been submitted
Thayer Martin Medium Base
Intended use
Recommended for selective isolation of Gonococci from pathological specimens.
Composition**
| Ingredients | g/L |
|---|---|
| Peptone, special | 23.000 |
| Starch | 1.000 |
| Sodium chloride | 5.000 |
| Agar | 13.000 |
Final pH (at 25°C) 7.0±0.2
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 21.0 grams in 450 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C. Aseptically add 50 ml of sterile lysed blood and rehydrated contents of one vial of Vitamino Growth Supplement (FD025) and V.C.N Supplement (FD023) or V.C.N.T Supplement (FD024). If desired GC Supplement with Antibiotics (FD021) can be used as a single supplement. Mix well before pouring into sterile Petri plates. If Hemoglobin (FD022) is used suspend 21.0 grams of Thayer Martin Medium Base in 250 ml distilled water. Heat to boiling to dissolve the medium completely. Prepare 250 ml of 2% hemoglobin solution. Sterilize separately by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C. Mix both and add the supplements as above.
Principle And Interpretation
Carpenter and Morton reported an improved medium to isolate Gonococci in 24 hours (1). Later on the efficiency of GC medium supplemented with haemoglobin and yeast concentrate was demonstrated for isolating gonococci (2). Subsequently Thayer and Martin Medium was developed for the primary isolation of Neisseria gonorrhoeae and Neisseria meningitidis from specimens containing mixed flora collected from throat, vagina, rectum and urethra (3,4). Thayer and Martin (4) used Vancomycin, Colistin and Nystatin. Martin and Lester (5) used an additional antibiotic Trimethoprim to make the medium selective.
Special peptone provides nutrients to the organisms while starch neutralizes the toxic fatty acids if present in the agar. Haemoglobin provides the X factor whereas the V factor (N.A.D.) is provided by the added supplement. Supplement (FD025) also supplies vitamins, amino acids, coenzymes etc. which enhances the growth of pathogenic Neisseria. Vancomycin and colistin inhibits gram-positive and gram-negative bacteria respectively (6). Nystatin inhibits fungi. This medium may inhibit Haemophilus species. Some strains of Capnocytophaga species may grow on this medium when inoculated with oropharyngeal specimens.
Type of specimen
Clinical samples : Throat, vaginal secretions, rectum and urethra
Specimen Collection and Handling:
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (4,5). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions :
In Vitro diagnostic Use only. For professional use only. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/ face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations :
- Due to nutritional variations and fastidious nature of organisms certain strains may show poor growth.
- Some strains of Capnocytophaga species may grow on this medium when inoculated with oropharyngeal specimens.
- Further biochemical identification is necessary for confirmation.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within expiry period when stored at the recommended temperature.
Quality Control
Appearance
Cream to yellow homogeneous free flowing powder
Gelling
Firm, comparable with 1.3% Agar gel.
Colour and Clarity of prepared medium
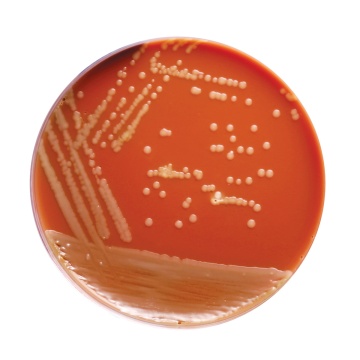
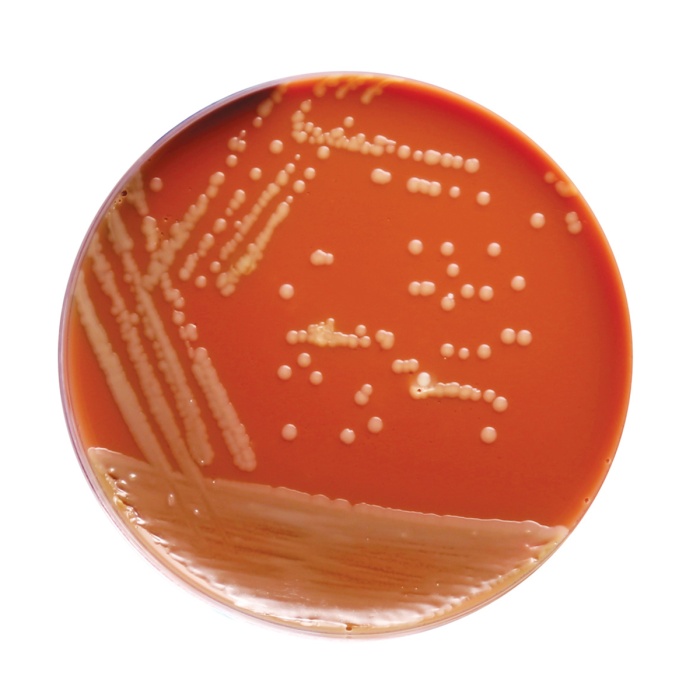
Basal Medium: Yellow coloured clear to slightly opalescent gel. After addition of haemoglobin or sterile lysed blood and supplements: chocolate coloured opaque gel forms in Petri plates.
Reaction
Reaction of 4.2% w/v aqueous solution at 25°C. pH: 7.0±0.2
pH
6.80-7.20
Cultural Response
Cultural characteristics observed with added sterile FO Growth Supplement (FD022), Vitamino Growth Supplement (FD025) and V.C.N. Supplement (FD023)/V.C.N.T. Supplement (FD024) after an incubation at 35-37°C for 18-48 hours.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| Escherichia coli ATCC 25922 (00013*) | >=104 | inhibited | 0% | |
| Neisseria gonorrhoeae ATCC 19424 | 50-100 | good-luxuriant | >=50% | small, grayish- white to colourless, mucoid |
| Neisseria meningitidis ATCC 13090 | 50-100 | good-luxuriant | >=50% | medium to large, blue- gray, mucoid |
| Proteus mirabilis ATCC 25933 | >=104 | inhibited | 0% |
Key: *Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle inorder to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (7,8).
Reference
- Carpenter and Morton, 1947, Proc. N.Y. State Assoc. Public Hlth. Labs., 27:58.
- Carpenter et al, 1949, Am. J. Syphil. Gonorrh. Vener. Dis., 33:164.
- Martin, Billings, Hackney and Thayer, 1967, Public Hlth. Rep., 82:361.
- Thayer J. and Martin J.E. Jr., 1966, Public Health Rep., 81:559.
- Martin J.E. Jr. and Lester A., 1971, HSMHA Hlth. Service Rep., 86(1):30.
- MacFaddin J., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. I, Williams and Wilkins, Baltimore.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | Thayer Martin Medium Base |
|---|---|
| SKU | M413 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Omata R. R. and Disraely M. N., 1956, J. Bacteriol., 72:677. 2.Power D. A. and Pelczar M. J., 1959, J. Bacteriol., 77 : 789 |