Your enquiry has been submitted
Your enquiry has been submitted
Xylose-Lysine Deoxycholate Agar, Granulated
Intended use
Recommended as a selective medium for the isolation and enumeration of Salmonella Typhi and other Salmonella species from pharmaceutical products in accordance with the microbial limit testing by harmonized methodology of USP/ EP/BP/JP/IP.
Composition**
| Ingredients | g/L |
|---|---|
| Xylose | 3.500 |
| L-lysine | 5.000 |
| Lactose monohydrate | 7.500 |
| Sucrose | 7.500 |
| Sodium chloride | 5.000 |
| Yeast extract | 3.000 |
| Sodium deoxycholate | 2.500 |
| Sodium thiosulphate | 6.800 |
| Ferric ammonium citrate | 0.800 |
| Phenol red | 0.080 |
| Agar | 13.500 |
| pH after heating (at 25°C) | 7.4±0.2 |
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 54.8 grams (the equivalent weight of dehydrated medium per litre) in 1000 ml purified/ distilled water. Heat with frequent agitation until the medium boils. DO NOT HEAT IN AN AUTOCLAVE. Transfer immediately to a water bath at 50°C. After cooling, pour into sterile Petri plates. It is advisable not to prepare large volumes, which will require prolonged heating and may produce precipitate.
Note: Slight precipitation in the medium may occur,which is inheritant property of the medium,and does not affect the performance of the medium.
Principle And Interpretation
Enterobacteriaceae is a family of gram-negative, non-spore-forming bacilli that contains more than 100 species of bacteria that normally inhabit the intestines of humans and animals. Members forming part of the normal intestinal microflora are referred to as coliforms. The clinically significant genera of Enterobacteriaceae include Cedecea, Citrobacter, Edwardsiella, Enterobacter, Escherichia, Ewingella, Hafnia, Klebsiella, Kluyvera, Proteus, Salmonella, Shigella and Yersinia (1).
The Salmonellae are the most complex of all the Enterobacteriaceae. Human Salmonella infections are most commonly caused by ingestion of food, water or milk, contaminated by human or animal excreta (2). A large number of media have been developed for the selective isolation and identification of enteric bacilli including Salmonella. Xylose Lysine Deoxycholate Agar is a selective as well as differential medium formulated by Taylor (3-7) for the isolation and identification of enteric pathogens especially Shigellae from stool samples. It is also used for pharmaceutical testing and non- sterile product testing for the detection (or absence) of Salmonella after enrichment in Rappaport Vassiliadis Salmonella Enrichment Broth (MH1491) in accordance with the harmonized method of USP/EP/BP/JP/IP (8-12).
Deoxycholate, ferric ammonium citrate and sodium thiosulphate are selective agents that inhibit gram-positive microorganisms. Essential nutrients, growth factors for growth of microorganism are provided by yeast extract. Xylose, sucrose and lactose are the fermentable sugars in this medium. Xylose is fermented by almost all the enteric bacteria except Shigellae, which enable the differentiation of Shigellae from Salmonellae. Salmonellae metabolize the xylose and decarboxylate lysine and thus change the pH to alkaline and mimic Shigellae reaction. However to prevent this reaction by lysine positive coliforms, lactose and sucrose are added in excess to produce acid and hence nonpathogenic H2S producers do not decarboxylate lysine. Sodium thiosulphate helps in reactivation of sulphur containing compounds and prevents the desiccation of these compounds during storage. It also forms the substrate for enzyme thiosulphate reductase, which breaks it to form H2S. Thiosulphate and ferric ammonium citrate are the H2S indicators in the medium. Sodium thiosulphate is also inactivator of halogens, mercurial and aldehyde and can minimize its toxicity in the testing sample, if any during microbial limit tests. Sodium chloride maintains the osmotic equilibrium in this medium. Phenol red is the pH indicator.
Degradation of fermentable sugars proceed concurrently and generates acids, which cause pH indicator to give various shades of colour, causing a color change in the colonies and in the medium from red to yellow on prolonged incubation. Hydrogen sulfide production results in colonies with black centers under alkaline conditions, which can be inhibited by acid production by carbohydrate fermentation. Alkaline condition causes the color of the medium to change back to red. This medium is an ideal medium for screening samples containing mixed flora of enteric pathogens as recovery of Salmonella and Shigella is not conspicuous by even profuse growth of other species (13,14).
Type of specimen
Pharmaceutical samples
Specimen Collection and Handling
For pharmaceutical samples, follow appropriate techniques for sample collection, processing as per guidelines (8-12). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations :
- Individual organisms differ in their growth requirement and may show variable growth patterns on the medium.
- Each lot of the medium has been tested for the organisms specified on the COA. It is recommended to users to validate the medium for any specific microorganism other than mentioned in the COA based on the user's unique requirement.
- Slight precipitation in the medium may occur, which is inheritant property of the medium,and does not affect the performance of the medium.
- Some Proteus strains may give red to yellow colouration with most colonies developing black centers, giving rise to false positive reactions. Non-enterics like Pseudomonas and Providencia may exhibit red colonies.
- S.Paratyphi A, S.Choleraesuis, S.Pullorum and S.Gallinarum may form red colonies without H2S, thus resembling Shigella species.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
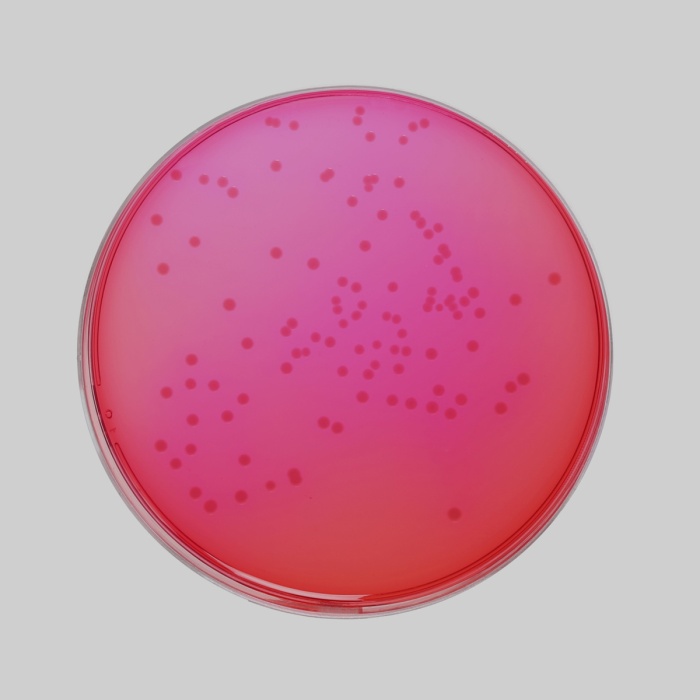
Appearance
Light yellow to pink coloured granular medium
Gelling
Firm, comparable with 1.35% Agar gel
Colour and Clarity of prepared medium
Red coloured clear to slightly opalescent gel forms in Petri plates
pH
7.20-7.60
Cultural Response
Growth Promotion is carried out in accordance with the harmonized method of USP/EP/BP/JP/IP. Cultural response was observed after an incubation at 30-35°C for specified time. Recovery rate is considered as 100% for bacteria growth on Soyabean Casein Digest Agar.
Growth promoting properties
Growth of microorganism comparable to that previously obtained with previously tested and approved lot of medium occurs at the specified temperature for not more than the shortest period of time specified inoculating <=100 cfu(at 30-35°C for <=18 hours).
Indicative properties
Colonies are comparable in appearance and indication reaction to those previously obtained with previously tested and approved lot of medium occurs for the specified temperature for a period of time within the range specified inoculating <=100cfu (at 30-35°C for 18-72 hours).
Inhibitory properties
No growth of the test microorganism occurs for the specified temp for not less than longest period of time specified inoculating >=100cfu (at 30-35°C for >= 72 hours).
Cultural Response
Cultural characteristics observed after incubation at 30-35 °C for 18-48 hours. Recovery rate is considered as 100% for bacteria growth on Soyabean Casein Digest Agar.
| Organism | Inoculum (CFU) | Growth | Observed Lot value (CFU) | Recovery | Colour of Colony | Incubation temperature |
|---|---|---|---|---|---|---|
| Growth Promoting + Indicative | ||||||
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | 25-100 | >=50% | red with black centres | 18-72 hrs |
| Salmonella Abony NCTC 6017 (00029*) | 50-100 | good-luxuriant | 25-100 | >=50% | red with black centres | 18-72 hrs |
| Additional Microbiological testing | ||||||
| Escherichia coli ATCC 8739 (00012*) | 50-100 | fair | 10-30 | 20-30% | yellow | 18-72 hrs |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | fair | 10-30 | 20-30% | yellow | 18-72 hrs |
| ## Proteus hauseri ATCC 13315 | 50-100 | good-luxuriant | 25-100 | >=50% | grey with black centres | 18 -72 hrs |
| Salmonella Paratyphi A ATCC 9150 | 50-100 | good-luxuriant | 25-100 | >=50% | red | 18-72 hrs |
| Salmonella Paratyphi B ATCC 8759 | 50-100 | good-luxuriant | 25-100 | >=50% | red with black centres | 18-72 hrs |
| Salmonella Enteritidis ATCC 13076 (00030*) | 50-100 | good-luxuriant | 25-100 | >=50% | red with black centres | 18-72 hrs |
| Salmonella Typhi ATCC 6539 | 50-100 | good-luxuriant | 25-100 | >=50% | red with black centres | 18-72 hrs |
| Shigella dysenteriae ATCC 13313 | 50-100 | good-luxuriant | 25-100 | >=50% | red | 18-72 hrs |
| Shigella flexneri ATCC 12022 (00126*) | 50-100 | fair-good | 15-40 | 30-40% | red | 18-72 hrs |
| Shigella sonnei ATCC 25931 | 50-100 | fair-good | 15-40 | 30-40% | red | 18-72 hrs |
| # Klebsiella aerogenes ATCC 13048 (00097*) | 50-100 | fair | 10-30 | 20-30% | yellow | 18-72 hrs |
| Enterobacter cloacae ATCC 13047 | 50-100 | fair | 10-30 | 20-30% | yellow | 18-72 hrs |
| Inhibitory properties | ||||||
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | >=10³ | inhibited | 0 | 0% | >=72 hrs | |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | >=10³ | inhibited | 0 | 0% | >=72 hrs | |
| Enterococcus faecalis ATCC 29212 (00087*) | >=10³ | inhibited | 0 | 0% | >=72 hrs | |
Key:(*) Corresponding WDCM numbers
(#) Formerly known as Enterobacter aerogenes ## Formerly known as Proteus vulgaris
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. For better performance. it is advised to store the plates at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (12,13).
| Product Name | Xylose-Lysine Deoxycholate Agar, Granulated |
|---|---|
| SKU | GMH031 |
| Product Type | Granulated |
| Physical Form | Granular |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Taylor W. L., 1965, Am. J. Clin. Pathol., 44:471-475. 2.Taylor W. L. and Harris B., 1965, Am. J. Clin. Pathol., 44:476. 3.Taylor W. L. and Harris B., 1967, Am. J. Clin. Pathol., 48:350. 4.Taylor W. L. and Schelhart B., 1967, Am. J. Clin. Pathol., 48:356. 5.Taylor W. L. and Schelhart B., 1968, Am. J. Clin. Pathol., 16:1387. 6.Taylor W. L. and Schelhart B., 1969, Appl. Microbiol., 18.393-395. 7.Chadwick P., Delisle G. H and Byer M., 1974, Can. J. Microbiol., 20, 1653-1664. 8.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 9.Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water andWastewater, 23rd ed., APHA, Washington, D.C. 10.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 11. Williams H., (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed., AOAC,Washington, D.C.1 2.FDA Bacteriological Analytical Manual, 2005, 18th Ed., AOAC, Washington, D.C. 13.Dunn C. and Martin W. J., 1971, Appl. Microbiol., 22, 17-22.1 4.Rollender M. A., Beckford O., Belsky R. D and Kostroff B. 1969, Am. J. Clin. Pathol., 51, 284-286. 15.Taylor W. L. and Schelhart B., 1969, Appl. Micro. 18, 1387-1392. 16.MacCarthy M. D., 1966, N. Z. J. Med. Lab. Technol., 20, 127-131. 17.Isenberg H. D., Kominos S., and Sigeal M., 1969, Appl Microbiol., 18, 656-659.1 20.Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.21. Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |