Your enquiry has been submitted
Your enquiry has been submitted
Oxytetra Glucose Yeast Agar Base, Granulated (OGYE Agar Base, Granulated)
Intended use
Recommended for isolation and enumeration of yeasts & moulds from milk and milk products. The composition and performance criteria are in accordance with ISO 1992, ISO/DIS 6611:2004.
Composition**
ISO 1992,ISO/DIS 6611:2004 Specification- Oxytetra Glucose Yeast Agar Base (OGYE Agar Base)
| Ingredients | g/L |
|---|---|
| Yeast extract powder | 5.000 |
| Dextrose (Glucose) | 20.000 |
| Agar | 10.000-15.000 |
| Final pH (at 25°C) | 6.6±0.2 |
GM639I- Oxytetra Glucose Yeast Agar Base (OGYE Agar Base),Granulated®
| Ingredients | g/L |
|---|---|
| Yeast extract | 5.000 |
| Dextrose (Glucose) | 20.000 |
| Agar | 12.000 |
| Final pH (at 25°C) | 6.6±0.2 |
**Formula adjusted, standardized to suit performance parameters
Supplements to be added after autoclaving
OxyT Selective Supplement-FD032 1 Vial
| *Ingredients | Concentration |
|---|---|
| Oxytetracycline | 50mg |
Directions
Suspend 18.5 grams in 500 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C and aseptically add reconstituted contents of one vial of OxyT Selective Supplement (FD032) or Genox Selective Supplement (FD131). Mix well and pour into sterile Petri plates.
Principle And Interpretation
Acidic media are not completely suitable for counting yeasts and moulds in foods since yeast cells, stressed by heat do not tolerate the acidic conditions necessary to inhibit bacterial contamination. Yeast and mould growth is often limited by the presence of acid-tolerant bacterial flora. Therefore it is evident that more active media and different selective agents are needed in order to deal with various kinds of foodstuffs, incubation conditions and types of microorganisms to be studied. Under certain conditions and when testing certain foods like milk and milk products, the use of oxytetracycline alone was not sufficient to obtain reliable yeast and mould counts. In particular, Mossel et al (1) observed that with proteinaceous foods heavily contaminated with gram-negative rods, it is necessary to use both oxytetracycline and gentamicin (FD131) in order to obtain complete inhibition of the contaminants.
OGYE Media were formulated by Mossel et al for the selective isolation and enumeration of yeast and moulds from foods (1,2). They found that addition of Oxytetra selective supplement to a neutral pH medium increased the recovery / count of yeast and moulds as compared to acidified medium. Psychotrophic yeasts can also be isolated when gentamicin is also incorporated into the medium (3). ISO Committee (4) has recommended OGYE Media with pH 6.6 ± 0.2 for isolation and enumeration of yeasts and moulds from milk and milk products.
Yeast extract provides essential growth nutrients. Dextrose acts as carbon and energy source. Low pH helps to reduce the bacterial flora. Oxytetracycline makes the medium more selective by inhibiting the growth of Lactobacilli encountered in milk and milk-products at low pH. The choice of a suitable media for enumeration of yeasts and moulds greatly depends on the nature of foodstuffs to be tested and the organisms that grow on them. These media remain bacteriostatic when inoculated with not greater than 1 ml of a 10-1 food dilution and incubation at 22°C. The number of yeasts or moulds is calculated per one gram or 1 ml of sample under investigation by multiplying the number of colonies with the dilution factor. Lactic acid bacteria are inhibited on this medium.
Type of specimen
Dairy samples
Specimen Collection and Handling:
For Dairy samples, follow appropriate techniques for sample collection and processing as per guidelines (5).
After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/ face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations :
- 1.Individual organisms differ in their growth requirement and may show variable growth patterns on the medium.
- 2. Each lot of the medium has been tested for the organisms specified on the COA. It is recommended to users to validate the medium for any specific microorganism other than mentioned in the COA based on the user's unique requirement.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Cream to light yellow coloured granular medium.
Gelling: Firm, comparable with 1.2% Agar gel.
Colour and Clarity of prepared medium: Light amber coloured clear to slightly opalescent gel forms in Petri plates
Reaction: Reaction of 3.7% w/v aqueous solution at 25°C. pH: 6.6±0.2
pH: 6.40-6.80
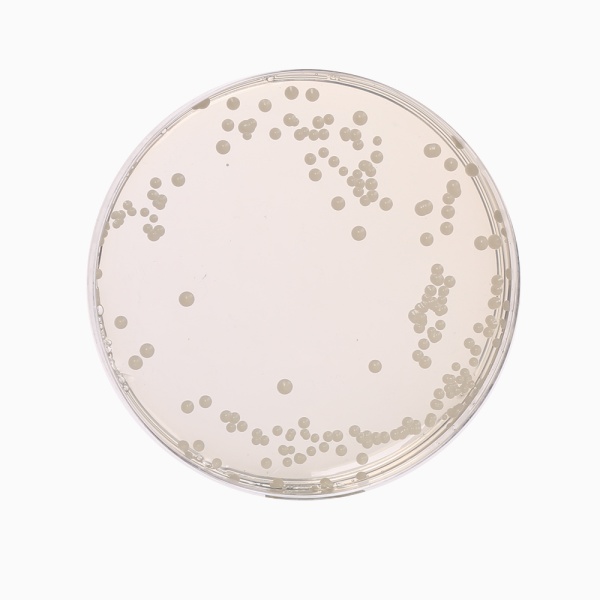
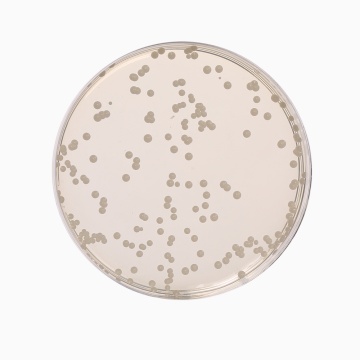
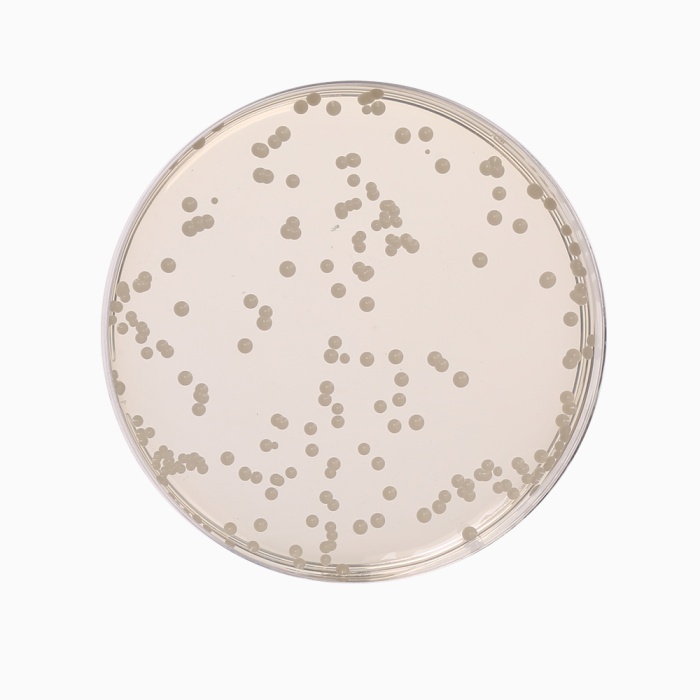
Cultural Response
Cultural characteristics observed with added 1 vial of OxyT Selective Supplement (FD032) or Genox Selective Supplement (FD131), after an incubation at 25-30°C after 2-5 days.
| Organism | Inoculum (CFU) | Growth | Recovery |
|---|---|---|---|
| #Aspergillus brasiliensis ATCC 16404 | 50-100 | good-luxuriant | >=50% |
| Candida albicans ATCC 10231 (00054*) | 50-100 | good-luxuriant | >=50% |
| Escherichia coli ATCC 25922 (00013*) | >=10³ | inhibited | 0% |
| Saccharomyces cerevisiae ATCC 9763 (00058*) | 50-100 | good-luxuriant | >=50% |
| Saccharomyces uvarum ATCC 9080 | 50-100 | good-luxuriant | >=50% |
Key: *Corresponding WDCM numbers. # Formerly known as Aspergillus niger
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle inorder to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (6,7).
| Product Name | Oxytetra Glucose Yeast Agar Base, Granulated (OGYE Agar Base, Granulated) |
|---|---|
| SKU | GM639I |
| Product Type | Granulated |
| Physical Form | Granular |
| Origin | Animal Free (Microbial) |
| Packaging type | HDPE |
| References | 1.Mossel D. A. A., Kleynen-Semmeling H. M., Vincentie H., Beerens H. and Catsaras M., 1970, J. Appl. Bacteriol., 33:4542.Mossel D. A. A., Visser M. and Mengerink W. H. J., 1962, Lab. Pract. 11:109. 3.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 4.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition. 5.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |