Your enquiry has been submitted
Your enquiry has been submitted
SS Agar (Salmonella Shigella Agar)
Shigella species#CC293D
Intended Use:
Recommended for the isolation of Salmonella and some Shigella species from pathological specimens, suspected foodstuffs etc.
Composition**
| Ingredients | g/L |
|---|---|
| Peptone | 5.000 |
| HM peptone B # | 5.000 |
| Lactose | 10.000 |
| Bile salts mixture | 8.500 |
| Sodium citrate | 10.000 |
| Sodium thiosulphate | 8.500 |
| Ferric citrate | 1.000 |
| Brilliant green | 0.00033 |
| Neutral red | 0.025 |
| Agar | 15.000 |
Final pH (at 25°C): 7.0±0.2
**Formula adjusted, standardized to suit performance parameters
# - Equivalent to Beef extract
Directions
Suspend 63.02 grams in 1000 ml purified /distilled water. Boil with frequent agitation to dissolve the medium completely. DO NOT AUTOCLAVE OR OVERHEAT. Overheating may destroy selectivity of the medium. Cool to about 50°C. Mix and pour into sterile Petri plates.
Principle And Interpretation
SS Agar medium is recommended as differential and selective medium for the isolation of Salmonella and Shigella species from pathological specimens (1) and suspected foodstuffs (2-5) and for microbial limit test (6). SS Agar is a moderately selective medium in which gram-positive bacteria are inhibited by bile salts, brilliant green and sodium citrate.
Peptone, HM peptone B provides nitrogen and carbon source, long chain amino acids, vitamins and essential growth nutrients. Lactose is the fermentable carbohydrate. Brilliant green, bile salts and thiosulphate selectively inhibit gram-positive and coliform organisms. Sodium thiosulphate is reduced by certain species of enteric organisms to sulphite and H2S gas and this reductive enzyme process is attributed by thiosulphate reductase. Production of H2S gas is detected as an insoluble black precipitate of ferrous sulphide, formed upon reaction of H2S with ferric ions or ferric citrate, indicated in the center of the colonies.
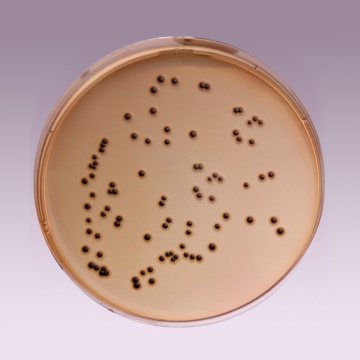
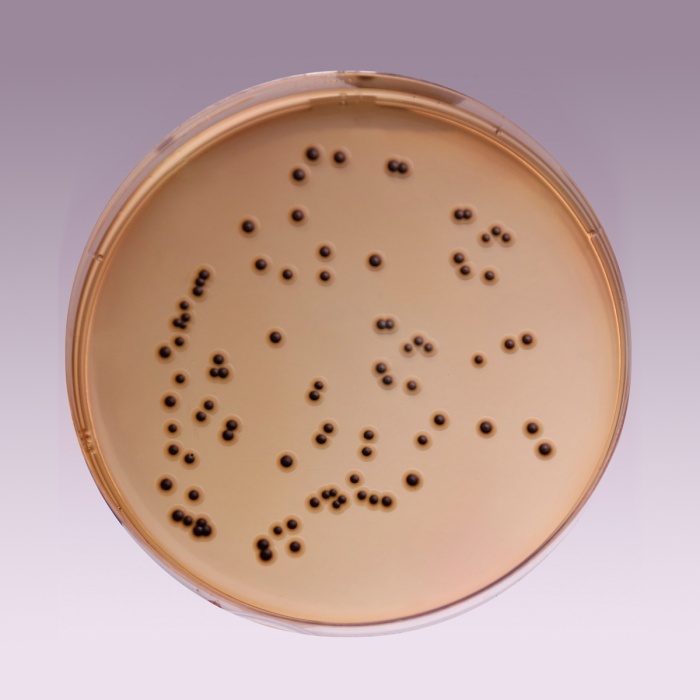
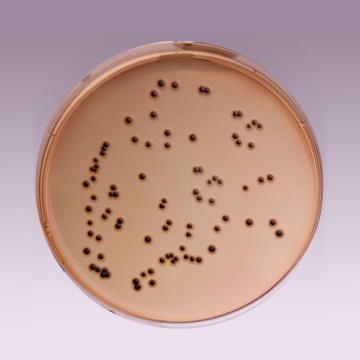
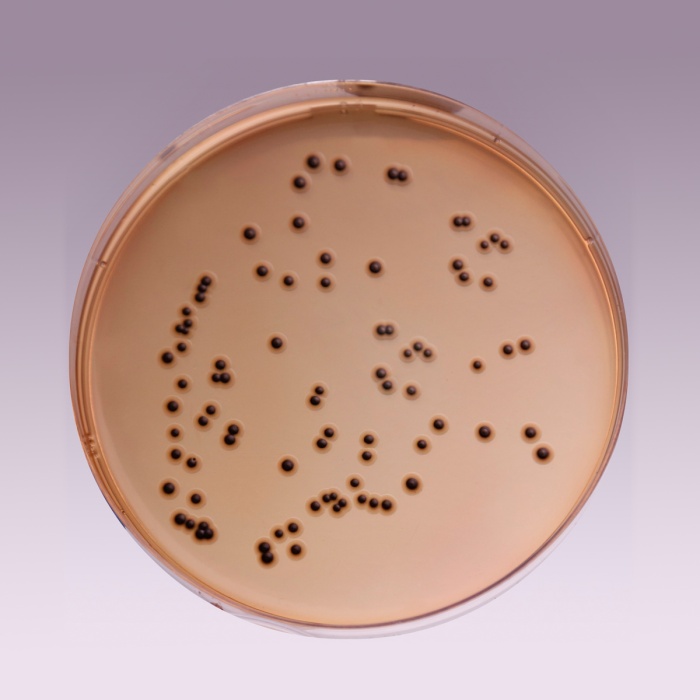
The high selectivity of Salmonella Shigella Agar allows the use of large inocula directly from faeces, rectal swabs or other materials suspected of containing pathogenic enteric bacilli. On fermentation of lactose by few lactose-fermenting normal intestinal flora, acid is produced which is indicated by change of colour from yellow to red by the pH indicator-neutral red. Thus these organisms grow as red pigmented colonies. Lactose non-fermenting organisms grow as translucent colourless colonies with or without black centers. Growth of Salmonella species appears as colourless colonies with black centers resulting from H2S production. Shigella species also grow as colourless colonies which do not produce H2S.
Type of specimen
Clinical: faeces, rectal swabs; Suspected food stuffs.
Specimen Collection and Handling
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (7,8).
For food and dairy samples, follow appropriate techniques for sample collection and processing as per guidelines (2-5). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
In Vitro diagnostic use. For professional use only. Read the label before opening the container. Wear protective gloves/ protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- The medium is highly selective and may be toxic to certain Salmonella or Shigella species. Hence it is recommended to use to inoculate plates of less inhibitory media parallel to SS Agar, such as Hektoen Enteric Agar (M467) or Deoxycholate Citrate Agar (M065) for easier isolation of Shigella species (3).
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance Light yellow to pink homogeneous free flowing powder
Gelling Firm, comparable with 1.5% Agar gel
Colour and Clarity of prepared medium Reddish orange coloured clear to slightly opalescent gel forms in Petri plates
Reaction Reaction of 6.3% w/v aqueous solution at 25°C. pH: 7.0±0.2
pH 6.80-7.20
Cultural Response Cultural characteristics observed after an incubation at 35-37°C for 18-24 hours.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| # Klebsiella aerogenes ATCC 13048 (00175*) | 50-100 | fair | 20-30% | cream pink |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | fair | 20-30% | pink with bile precipitate |
| Salmonella Choleraesuis ATCC 12011 | 50-100 | good-luxuriant | >=50% | colourless with black centre |
| Salmonella Typhi ATCC 6539 | 50-100 | good-luxuriant | >=50% | colourless with black centre |
| Enterococcus faecalis ATCC 29212 (00087*) | 50-100 | none-poor | <=10% | colourless |
| Proteus mirabilis ATCC 25933 | 50-100 | fair-good | 30-40% | colourless, may have black centre |
| Shigella flexneri ATCC 12022 (00126*) | 50-100 | good | 40-50% | colourless |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | good-luxuriant | >=50% | colourless with black centre |
| Salmonella Enteritidis ATCC 13076 (00030*) | 50-100 | good-luxuriant | >=50% | colourless with black centre |
Key: *Corresponding WDCM numbers. # Formerly known as Enterobacter aerogenes
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (7,8).
Reference
- Lennette and others (Eds.), 1985, Manual of Clinical Microbiology, 4th ed., ASM, Washington, D.C.
- Lipps WC, Braun-Howland EB, Baxter TE, eds. Standard methods for the Examination of Water and Wastewater, 24th ed. Washington DC:APHA Press; 2023.
- Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C.
- Williams S., (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed.,AOAC, Washington, D.C.
- The United States Pharmacopoeia-National Formulatory (USP-NF), 2022.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | SS Agar (Salmonella Shigella Agar) |
|---|---|
| SKU | M108 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1.Lennette and others (Eds.), 1985, Manual of Clinical Microbiology, 4th ed., ASM, Washington, D.C. 2.Downes F. P. and Ito K., (Eds.), 2001, Compendium of Methods for the Microbiological Examination of Foods, 4th Ed.,APHA, Washington, D.C. 3.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 4.Eaton A. D., Clesceri L. S., Rice E. W., and Greenberg A. W., (Eds.), 2005, Standard Methods for the Examination of Waterand Wastewater, 21st Ed., APHA, Washington, D.C. 5.Williams S., (Ed.), 2005, Official Methods of Analysis of the Association of Official Analytical Chemists, 19th Ed., AOAC,Washington, D.C. 6.The United States Pharmacopoeia, 2006, USP29/NF24, The United States Pharmacopoeial Convention. Rockville, MD. 7.MacFaddin J., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. I, Williams andWilkins, Baltimore. 9.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual ofClinical Microbiology, 11th Edition. Vol. 1. |