Your enquiry has been submitted
Your enquiry has been submitted
Nutrient Agar, pH 7.0
Intended Use
Recommended for the cultivation of Salmonella species.
Composition
| Ingredients | Gms / Litre |
|---|---|
| Peptone | 5.000 |
| HM extract # | 3.000 |
| Agar | 15.000 |
| Final pH (at 25°C) | 7.0±0.2 |
**Formula adjusted, standardized to suit performance parameters
# Equivalent to Meat extract
Directions
Suspend 23 grams in 1000 ml purified/distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 45-50°C. If desired, the medium can be enriched with 5 - 10% v/v sterile defibrinated blood. Mix well and Pour into Sterile Petri plates
Principle And Interpretation
Nutrient Agar is a basic culture medium used for maintenance or to check purity of subcultures prior to biochemical or serological tests from water (1) and Dairy (7). Many bacteria have the optimum pH growth range of 6.6 to 7.0. This medium may be used as slants or plates for routine work with non-fastidious organisms. Wetmore and Gochenour (8) maintained cultures of Malleomyces and Pseudomonas on Nutrient Agar to which glycerol was added. Greenberg and Cooper (2) employed this medium in cultivation of Staphylococci for the preparation of vaccines and antigens. Nutrient Agars have relatively simple formulation which provides the necessary nutrients for the growth of many microorganisms which are not very fastidious. HM extract contains vitamins, organic nitrogen compounds, salts and little carbohydrates (5). Peptone provide amino acids and long chain peptides for the organisms.
Type of specimen
Food sample
Specimen Collection and Handling
For food samples, follow appropriate techniques for sample collection and processing as per guidelines (6). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidleines should be followed while handling specimens. Saftey guidelines may be referred in individual safety data sheets.
Limitations
- Due to variable nutritional requirements, some strains show poor growth on this medium.
- Further biochemical and serological testing is required for complete identification.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Cream to yellow homogeneous free flowing powder
Gelling: Firm, comparable with 1.5% Agar gel
Colour and Clarity of Prepared medium: Yellow coloured clear to slightly opalescent gel forms in Petri plates
Reaction: Reaction of 2.3% w/v aqueous solution at 25°C. pH: 7.0±0.2
pH: 6.80-7.20
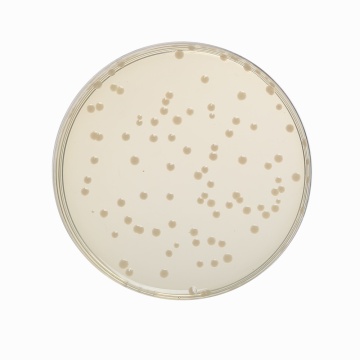
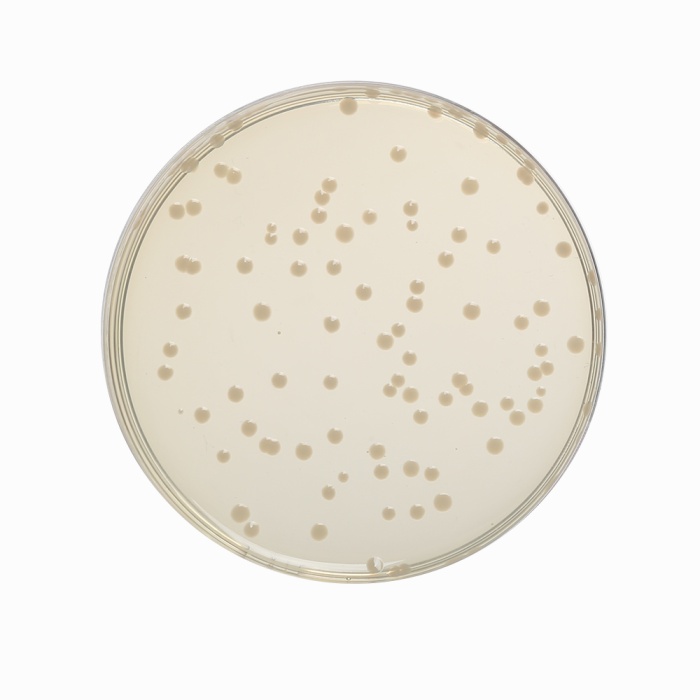
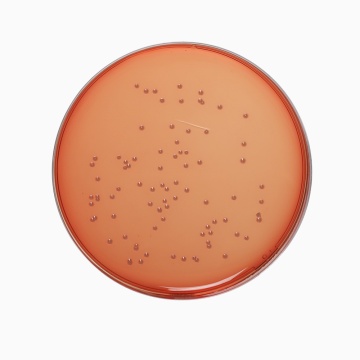
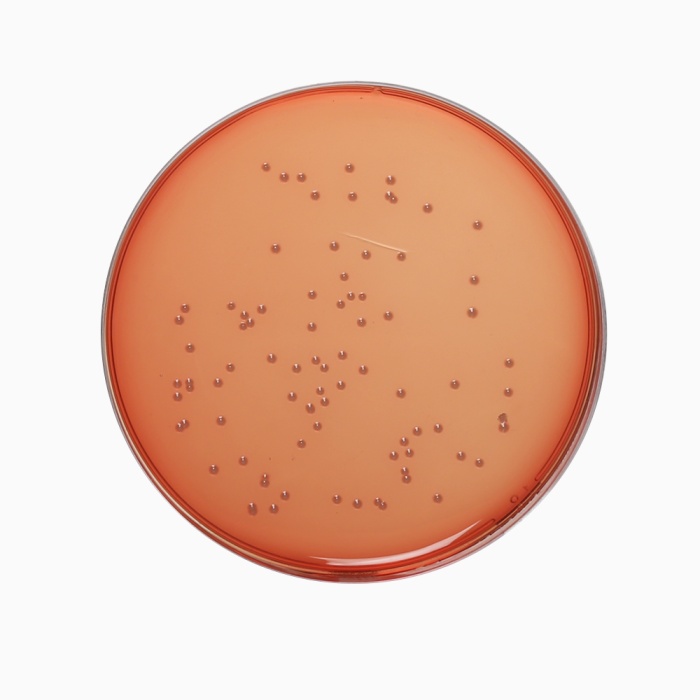
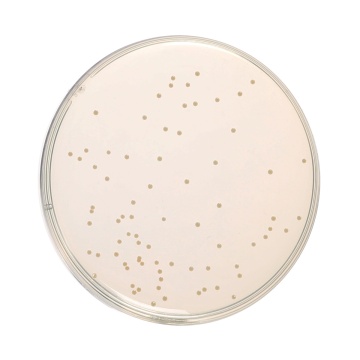
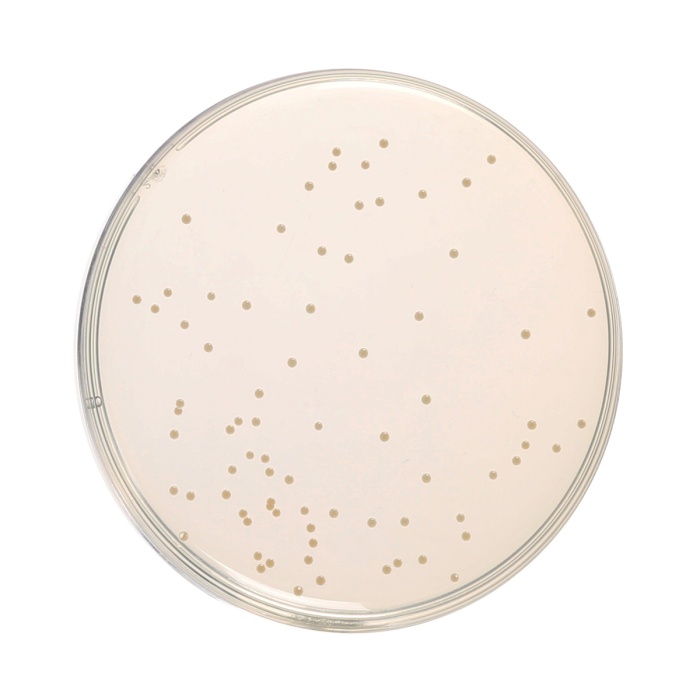
Cultural Response
Cultural characteristics observed after an incubation at 35-37°C for 18-24 hours.
| Organism | Inoculum (CFU) | Growth | Recovery |
|---|---|---|---|
| Enterococcus faecalis ATCC 29212 (00087*) | 50-100 | luxuriant | >=70% |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | luxuriant | >=70% |
| Escherichia coli ATCC 8739 (00012*) | 50-100 | luxuriant | >=70% |
| Salmonella Enteritidis ATCC 13076 (00030*) | 50-100 | luxuriant | >=70% |
| Salmonella Typhi ATCC 6539 | 50-100 | luxuriant | >=70% |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | >=70% |
| Shigella flexneri ATCC 12022 | 50-100 | luxuriant | >=70% |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | 50-100 | luxuriant | >=70% |
| Yersinia enterocolitica ATCC 9610 (00038*) | 50-100 | luxuriant | >=70% |
| Yersinia enterocolitica ATCC 23715 (00160*) | 50-100 | luxuriant | >=70% |
Key: (*) Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle inorder to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use.Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (3,4).
Reference
- Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water and Wastewater, 23rd ed., APHA, Washington, D.C.
- Greenberg and Cooper, 1960, Can. Med. Assn. J., 83:143.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
- Pelczar, Chan and Kreig, 1986, Microbiology, 5th ed., McGraw-Hill Book Company, New York.
- Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Standard Methods for the Examination of Dairy Products, 1978, 14th ed., APHA, Washington D.C.
- Wetmore and Gochenour, 1956, J. Bact., 72:79.
| Product Name | Nutrient Agar, pH 7.0 |
|---|---|
| SKU | M561A |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water andWastewater, 23rd ed., APHA, Washington, D.C. 2.Standard Methods for the Examination of Dairy Products, 1978, 14th ed., APHA, Washington D.C. 3.Wetmore and Gochenour, 1956, J. Bact., 72:79. 4.Greenberg and Cooper, 1960, Can. Med. Assn. J., 83:143. 5.Pelczar, Chan and Kreig, 1986, Microbiology, 5th ed., McGraw-Hill Book Company,New York. 6.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 7.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2001, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 8.Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition. 9.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |
| Customized Product Available | No |