Your enquiry has been submitted
Your enquiry has been submitted
EMB Agar Base
Intended Use
A basal medium to which different carbohydrates and other test substances may be added for differentiation and study of various enteric bacteria.
Composition**
| Ingredients | g / L |
|---|---|
| Peptone | 10.000 |
| Dipotassium hydrogen phosphate | 2.000 |
| Eosin - Y | 0.400 |
| Methylene blue | 0.065 |
| Agar | 15.000 |
Final pH (at 25°C): 7.3±0.2
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 27.46 grams in 1000 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Add desired carbohydrate or other test substance in desired concentration. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. DO NOT OVERHEAT. Cool to 45-50°C and shake the medium in order to oxidize the methylene blue (i.e. restore its blue colour) and to suspend the precipitate, which is an essential part of the medium. Mix well and pour into sterile Petri plates.
Precaution : Store the medium away from light to avoid photooxidation.
Principle And Interpretation
Levine EMB Agar was developed by Levine (1,2) and is used for the differentiation of Escherichia coli and Enterobacter aerogenes and also for the rapid identification of Candida albicans. This medium is recommended for the detection, enumeration and differentiation of members of the coliform group by American Public Health Association (3-5). Some gram-positive bacteria such as faecal Streptococci, yeasts grow on this medium and form pinpoint colonies. EMB Agar Base is a modification of EMB Agar, Levine without lactose. This facilitates the use of the medium as a basal agar to which desired carbohydrates could be added to differentiate between various enteric bacteria.
Eosin-Y and methylene blue make the medium slightly selective and inhibit certain gram-positive bacteria. These dyes differentiate between lactose fermenters and nonfermenters. The ratio of eosin-methylene blue is adjusted to approximately 6:1. Coliforms produce purplish black colonies due to uptake of methylene blue-eosin dye complex, when the pH drops. The dye complex is absorbed into the colony. Non-fermenters probably raise the pH of surrounding medium by oxidative de-amination of protein, which solubilizes the methylene blue-eosin complex resulting in formation of colourless colonies (6). Peptone serves as source of carbon, nitrogen, and other essential growth nutrients. Eosin-Y and methylene blue serve as differential indicators. Phosphate buffers the medium.
Type of specimen
Clinical samples - faeces; Water samples.
Specimen Collection and Handling
The test sample can be directly streaked on the medium plates. Inoculated plates should be incubated, protected from light. However standard procedures should be followed to obtain isolated colonies. A non-selective medium should be inoculated in conjunction with EMB Agar.
Warning and Precautions
In Vitro diagnostic use. For professional use only. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Confirmatory tests should be further carried out for identification of isolated colonies.
- Store the plates away from light to avoid photo-oxidation
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance: Light pink to purple homogeneous free flowing powder
Gelling: Firm, comparable with 1.5% Agar gel
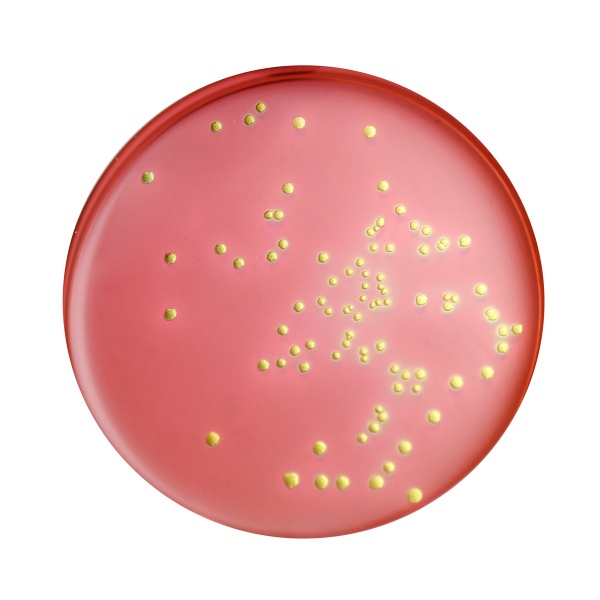
Colour and Clarity of prepared medium: Reddish purple coloured, opalescent gel with greenish cast and finely dispersed precipitate forms in Petri plates
Reaction: Reaction of 2.75% w/v aqueous solution at 25°C. pH : 7.3±0.2
pH: 7.10-7.50
Cultural Response: Cultural characteristics observed with added carbohydrate after an incubation at 35-37°C for 18-24 hours (Fungal cultures incubated at 25-30°C for 24-48 hours).
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| Escherichia coli ATCC 25922 (00013*) | 50-100 | luxuriant | >=50% | blue-black with green metallic sheen |
| # Klebsiella aerogenes ATCC 13048 (00175*) | 50-100 | good-luxuriant | >=50% | pink-red |
| Enterococcus faecalis ATCC 29212 (00087*) | 50-100 | non-poor | <=10% | colourless |
| Pseudomonas aeruginosa ATCC 27853 (00025*) | 50-100 | luxuriant | >=50% | colourless |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | >=50% | colourless |
| Saccharomyces cerevisiae ATCC 9763 (00058*) | 50-100 | none-poor | <=10% | cream |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | 50-100 | none-poor | <=10% | colourless |
| Candida albicans ATCC 10231 (00054*) | 50-100 | luxuriant (incubated in 10% CO2) | >=50% | colourless |
Key : (*) Corresponding WDCM numbers. (#) Formerly known as Enterobacter aerogenes
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C and away from sunlight. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with sample must be decontaminated and disposed of in accordance with current laboratory techniques (7,8).
Reference
- Levine M., 1921, Bull. 62, Iowa State College Engr. Exp. Station.
- Levine M., 1918, J. Infect. Dis., 23:43.
- Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water and Wastewater, 23rd ed., APHA, Washington, D.C.
- Marshall R. (Ed.), 1992, Standard Methods for the Examination of Dairy ,, Products, 16th ed., APHA Inc., New York.
- Salfinger Y., and Tortorello M.L., 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Howard B. J., 1994, Clinical and Pathogenic Microbiology, 2nd Ed., Mosby Year Book, Inc
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
| Product Name | EMB Agar Base |
|---|---|
| SKU | M301 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Moffett M., Young D. and Stuart R. D., 1948, Brit, Med. J., 2:241. 2.Stuart S. D., Toshach S. R. and Patsula T. M., 1954, Can. J. Public Health, 45:73. |
| Customized Product Available | No |