Your enquiry has been submitted
Your enquiry has been submitted
Reinforced Clostridial HiVeg® Agar
Composition
| Ingredients | MV154 Grams/Litre | MV443 Grams/Litre |
|---|---|---|
| HiVeg® hydrolysate | 10.00 | 10.00 |
| HiVeg® extract | 10.00 | 10.00 |
| Yeast extract | 3.00 | 3.00 |
| Dextrose | 5.00 | 5.00 |
| Sodium chloride | 5.00 | 5.00 |
| Sodium acetate | 3.00 | 3.00 |
| Starch, soluble | 1.00 | 1.00 |
| L-Cysteine hydrochloride | 0.50 | 0.50 |
| Agar | 13.50 | 0.50 |
Final pH (at 25°C) 6.8± 0.2
** Formula adjusted, standardized to suit performance parameters
Product Profile
Vegetable based (Code MV)
- MV154/MV443
- HiVeg® hydrolysate
- HiVeg® extract
Animal based (Code M)
- M154/M443
- Casein enzymic hydrolysate
- Beef extract
Recommended for : Cultivation and enumeration of Clostridium and other anaerobes.
Reconstitution :
- (MV154) : 51.0 g/l
- (MV443) : 38.0 g/l
Quantity on preparation (500g) :
- (MV154) : 9.80 L
- (MV443) : 13.15 L
(100g) :
- (MV154) : 1.96 L
- (MV443) : 2.63 L
pH (25°C) : 6.8± 0.2
Supplement : None
Sterilization : 115°C / 15 minutes.
Storage: Dry Medium - Below 30°C, Prepared Medium 2 - 8°C.
Directions
Suspend 51 grams of MV154 or 38 grams of MV443 in 1000 ml distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 10 lbs pressure (115°C) for 15 minutes.
Principle and Interpretation
Reinforced Clostridial HiVeg® Agar/ Broth is specially developed using HiVeg® hydrolysate and HiVeg® extract to avoid BSE/TSE risks associated with animal origin peptones and extracts. Reinforced Clostridial HiVeg® Agar / Broth Media are the modifications of Reinforced Clostridial Media which are formulated by Hirsch and Grinsted (1). It can be used to initiate growth from small inocula and to obtain the highest viable count of Clostridia. This medium like the conventional medium can be used for diluting an inoculum of vegetative cells of Clostridium perfringens (2) as suggested by Barnes and Ingram or can be used in studies of spore forming anaerobes, especially Clostridium butyricum in cheese, also for the enumeration of Clostridia in tube dilution counts and for preparation of plates for isolation (3). Other spore forming anaerobes, Streptococci and Lactobacilli also grow in these media. These are enriched but nonselective media.
HiVeg® hydrolysate, yeast extract, HiVeg® extract and starch, provide all the necessary nutrients for the growth of Clostridia. Dextrose is a fermentable carbohydrate in the medium while sodium chloride maintains osmotic equilibrium. Cystine hydrochloride is the reducing agent whereas sodium acetate acts as buffer. These media can be made selective by addition of 15-20 mg Polymyxin B per litre of media (1).
Quality Control
Appearance of Powder
Light yellow coloured may have slightly greenish tinge, homogeneous, free flowing powder.
Gelling
Firm, comparable with 1.35% Agar gel of MV154.
Colour and Clarity
Light yellow coloured, clear to slightly opalescent gel forms in petri plates, clear solution in tubes.
Reaction
Reaction of 5.1% w/v of MV154 or 3.8% w/v of MV443 aqueous solution is pH 6.8± 0.2 at 25°C.
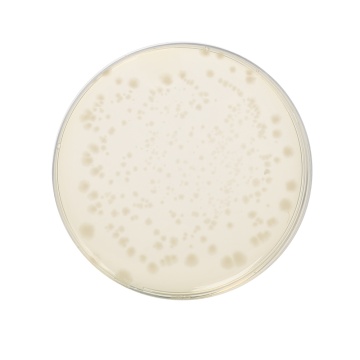
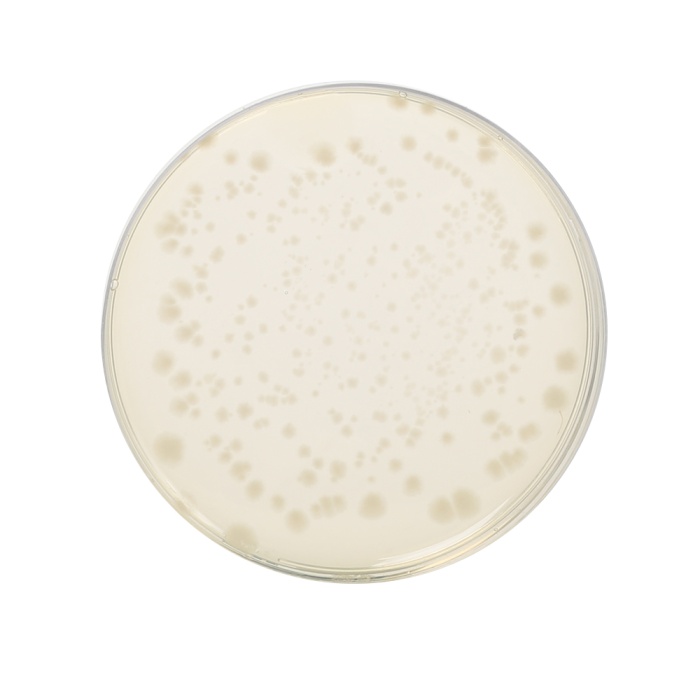
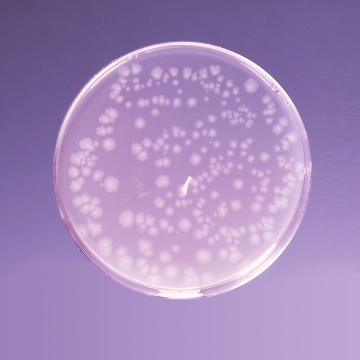
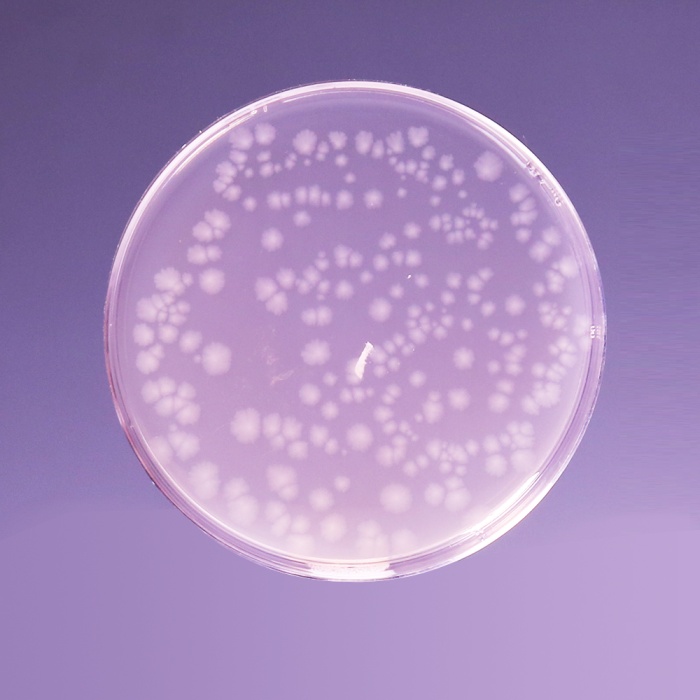
Cultural Response
Cultural characteristics observed after an incubation at 35-37°C for 40-48 hours, in an anaerobic atmosphere.
| Organisms (ATCC) | Inoculum (CFU) | Growth | Recovery |
|---|---|---|---|
| Bacteroides fragilis (23745) | 10²-10³ | good to luxuriant | >70% |
| Bacteroides vulgatus (8482) | 10²-10³ | good to luxuriant | >70% |
| Clostridium butyricum (9690) | 10²-10³ | good to luxuriant | >70% |
| Clostridium perfringens (13124) | 10²-10³ | good to luxuriant | >70% |
References
- Hirsch and Grinsted, 1954, J. Dairy Res., 21:101.
- Barnes and Ingram, 1956, J. Appl. Bact., 19:117.
- Lewis and Angelotti (Eds.), 1964, Examination of Foods for Enteropathogenic and Indicator Bacteria, Dept. of HEW, PHS Publication, 1142, Washington.
| Product Name | Reinforced Clostridial HiVeg® Agar |
|---|---|
| SKU | MV154 |
| Product Type | HiVeg™ |
| Physical Form | Powder |
| Origin | Animal Free (Veg) |
| Packaging type | HDPE |
| References | 1.American Public Health Association, Standard Methods for the Examination of Dairy Products, 1978, 14th Ed., Washington D.C. 2.Barnes E. M., Despaul J. E. and Ingram M., 196 3.J. Appl. Bacteriol. 26:41 3.Barnes E. M. and Ingram J. E., 195 6.J. Appl. Bacteriol. 19:11 4.Hirsch A. and Grinstead C., 1954, J. Dairy Res. 21:10 5.Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition. 6.Jorgensen,J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock.,D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1. 7.Salfinger Y., and Tortorello M.L. Fifth (Ed.),2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C. 8.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. |