MicroQuant
×
MicroQuant Microbial Reference Material: Precision & Trust
A single-use, cryopreserved, passage-zero reference material for streamlined microbial quality control in pharmaceutical testing, engineered under ISO 17034 certification.Key Features & Benefits:
- Accurate CFU Counts: Available in high- (10⁷-10⁸ CFU/vial) and low-titer (100-1,000 CFU/vial) formats.
- Format: Single-use pellets that rehydrate quickly (in ~1 minute).
- Stability: Usable for 8 hours post-rehydration (at 2-8°C) and fridge-stable for 6-12 months.
- Certified Quality: ISO 17034 accredited Reference Material, featuring original ATCC, passage-zero strains.
- Compliance: Supports global standards like USP, EP, and JP methods.
- Packaging: Dual-pack format (5 pellets + 5 buffer vials).
Ready in under a minute: MicroQuant rehydrates and is ready for plating in less than a minute, saving significant time in QC workflows.
Convenient storage: Its stable format stores easily at 2-8°C, eliminating the need for freezer storage, which reduces equipment costs and protects microbial integrity.
Original source strains: Uses ATCC-authenticated, passage-zero strains for full traceability, consistent performance, and confidence under ISO 17034 compliance.
https://www.atcc.org/microbe-products/applications/quality-control/microquant#t=productTab&numberOfResults=24
×
HepatoXcell Primary Human Hepatocytes for Drug Development and Toxicity Testing
Primary human hepatocytes are the gold standard for in vitro liver models, offering high predictive value for drug metabolism and toxicity studies.ATCC Hepatocytes (HepatoXcell Pro) Advantages:
- Premium Plate-ability: HepatoXcell™ Pro 7-day plateable hepatocytes provide extended viability for longer-term ADME-Tox and high-content screening assays.
- Comprehensive Characterization: Rigorous testing ensures consistency, including enzyme activity, gene expression, and metabolic function.
- Batch-specific DMPK Data: Downloadable Certificates of Analysis (COAs) include key DMPK metrics (metabolite profiling, induction, transporter activity).
- Expert Support: Dedicated technical team for consultation, training, and on-site support.
- Easy Lot Selection: Use the lot selection tool based on donor demographics and enzyme activities.
- Detailed Protocols: Tailored thawing, plating, media, and maintenance protocols provided.
- Global Shipping: Reliable, temperature-monitored liquid nitrogen shipping worldwide.
×
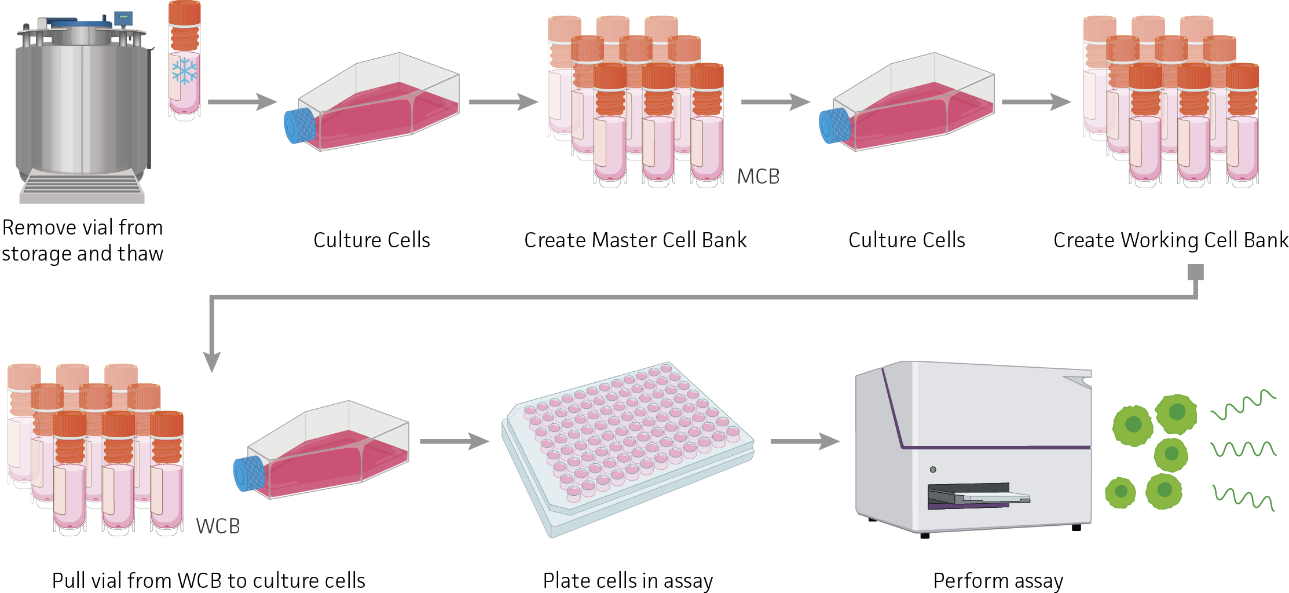
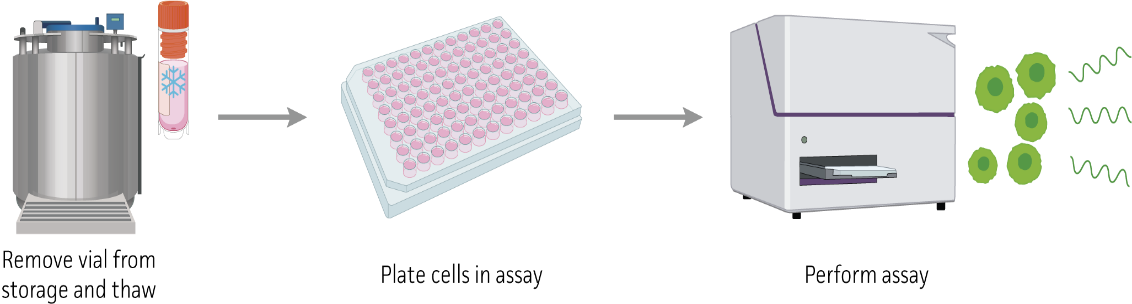
ThawReady Assay Ready Cells: From Frozen to Data in 1 Day
ATCC's ThawReady Assay Ready Cells eliminate the lengthy cell expansion process needed for synchronized cell stock, saving you months in your drug discovery and development workflow.

Key Benefits:
- Eliminate lengthy cell expansion (saves cost, time, and lab space).
- Ready within hours of thawing.
- Scalable for high-throughput assays.
You simply thaw, plate, and go.
https://www.atcc.org/cell-products/cell-models/assay-ready-cells#t=productTab&numberOfResults=24-
ADDENDUM TO ATCC MTA For Use of Certain ATCC® Materials in Screening Applications


