Your enquiry has been submitted
Your enquiry has been submitted
Brilliant Green Agar Base, Modified
MPN Confirmed#CC293D
Intended Use
Recommended for selective isolation of Salmonellae other than Salmonella Typhi from faeces, food, dairy products.
Composition**
| Ingredients | g/L |
|---|---|
| Proteose peptone | 10.000 |
| Yeast extract | 3.000 |
| Lactose | 10.000 |
| Sucrose | 10.000 |
| Sodium chloride | 5.000 |
| Phenol red | 0.080 |
| Brilliant green | 0.0125 |
| Agar | 20.000 |
Final pH (at 25°C): 6.9±0.2
**Formula adjusted, standardized to suit performance parameters
Directions
Suspend 29.0 grams in 500 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. AVOID OVERHEATING. Cool to 45-50°C. For more selectivity, aseptically add rehydrated contents of 1 vial of S Selective Supplement (FD068). Mix well before pouring into sterile Petri plates.
Principle And Interpretation
Salmonella species cause many types of infections, from mild self-limiting gastroenteritis to life threatening typhoid fever. The most common form of Salmonella disease is self-limiting gastroenteritis with fever lasting less than 2 days and diarrhoea lasting less than 7 days. Brilliant Green Agar Base, Modified, as a primary plating medium for isolation of Salmonella species was first described by Kristensen et. al. (1) and further modified by Kauffmann (2). Brilliant Green Agar is also recommended by APHA (3,4) FDA (5) and described in EP, BP and IP (6,7,8).
This medium contains brilliant green, which inhibits growth of majority of Gram-negative and Gram-positive bacteria. Salmonella Typhi, Shigella species Escherichia coli, Pseudomonas species, Staphylococcus aureus are mostly inhibited. Clinical specimens can be directly plated on this medium. However, being highly selective, it is recommended that this medium should be used along with a less inhibitory medium to increase the chances of recovery. Often cultures enriched in Selenite or Tetrathionate Broth is plated on Brilliant Green Agar along with Bismuth Sulphite Agar, SS Agar, MacConkey Agar.
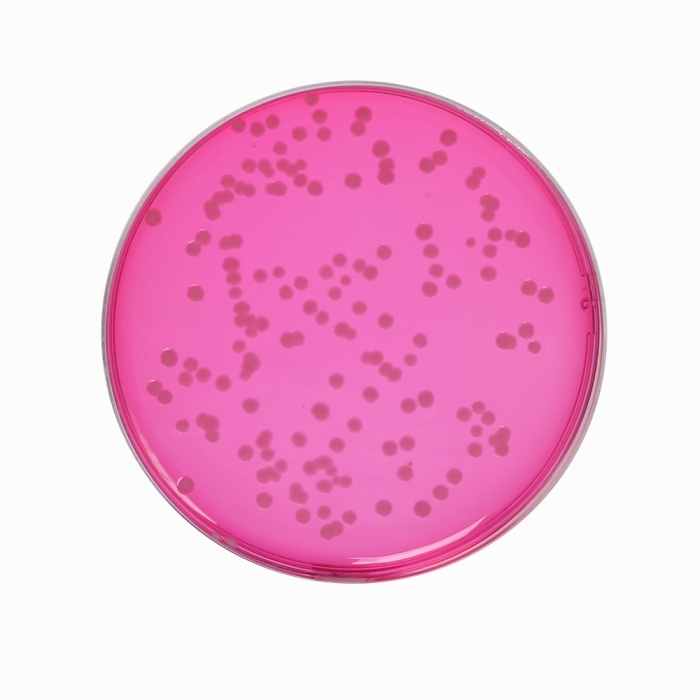
The medium contains proteose peptone and yeast extract as sources of carbon, nitrogen, vitamins, amino acids and essential nutrients. The two sugars namely lactose and sucrose serve as energy sources. Fermentation of lactose and/or sucrose in the medium results in the formation of acidic pH which is detected by phenol red indicator. Sodium chloride maintains the osmotic equilibrium. Brilliant green helps to inhibit the contaminating microflora. The medium can further supplemented with sulphaacetamide (1g/l) and sodium mandelate (0.25g/l) to inhibit contaminating microorganisms when the sample is suspected to contain large number of competing organisms along with Salmonella species. Non-lactose fermenting bacteria develop white to pinkish red colonies within 18 - 24 hours of incubation.
Type of specimen
Clinical: Faeces; Foodstuffs & dairy samples; Water samples; Pharmaceutical samples.
Specimen Collection and Handling
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (9,10).
For food and dairy samples, follow appropriate techniques for sample collection and processing as per guidelines (3,11,12,13).
For water samples, follow appropriate techniques for sample collection, processing as per guidelines and local standards (5) .
Warning and Precautions
In Vitro diagnostic use. For professional use only. Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Though this medium is selective for Salmonella other species of Enterobacteriaceae may grow.
- Salmonella Typhi and Shigella species may not grow on this medium.
- Moreover Proteus, Pseudomonas and Citrobacter species may mimic enteric pathogens by producing small red colonies.
- Further confirmation has to be carried out on presumptive Salmonella isolates.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance Light yellow to light pink homogeneous free flowing powder
Gelling Firm, comparable with 2.0% agar gel.
Colour and Clarity of prepared medium Greenish brown clear to slightly opalescent gel forms in Petriplates
Reaction Reaction of 5.8% w/v aqueous solution at 25°C. pH : 6.9±0.2
pH 6.70-7.10
Cultural Response Cultural response was carried out after an incubation at 30-35°C for 24-48 hours. Recovery rate is considered as 100% for bacteria growth on Soyabean Casein Digest Agar.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of Colony |
|---|---|---|---|---|
| Escherichia coli ATCC 25922 (00013*) | 50-100 | none-poor | 0-10% | yellowish green |
| Escherichia coli ATCC 8739 (00012*) | 50-100 | none-poor | 0-10% | yellowish green |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | >=104 | inhibited | 0% | |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | >=104 | inhibited | 0% | |
| Salmonella Typhi ATCC 6539 | 50-100 | fair-good | 30-40% | reddish pink |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | good-luxuriant | >=50% | pinkish white |
| Salmonella Enteritidis ATCC 13076 (00030*) | 50-100 | luxuriant | >=50% | pinkish white |
| Salmonella Abony NCTC 6017 (00029*) | 50-100 | good-luxuriant | >=50% | pinkish white |
Key: *Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 2-8°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (9,10).
Reference
- Kauffman F., 1935, Seit F. Hyg. 177:26.
- Kristensen M., Lester V, and Jurgens A., 1925, Brit.J.Exp.Pathol., 6:291.
- Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Standard Methods for the Microbiological Examination of Dairy Products, 1995, 19th Ed, APHA, Washington, D.C.
- Lipps WC, Braun-Howland EB, Baxter TE,eds. Standard methods for the Examination of Water and Wastewater, 24th ed. Washington DC:APHA Press; 2023.
- Indian Pharmacopoeia, 2018, Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare Government of India.
- The British Pharmacopoeia, 2022, Medicines and Healthcare products Regulatory Agency.
- European Pharmacopoeia, 2022, 10 th volume, European Directorate for the quality of medicines & Healthcare.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
- American Public Health Association, Standard Methods for the Examination of Dairy Products, 1978, 14th Ed., Washington D.C.
- Bacteriological Analytical Manual, 5th Ed, 1978, AOAC, Washington D.C.
- Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed., APHA Inc., Washington, D.C.
| Product Name | Brilliant Green Agar Base, Modified |
|---|---|
| SKU | M016 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Kristensen M., Lester V, and Jurgens A., 1925, Brit.J.Exp.Pathol.,6:291. 2.Kauffman F., 1935, Seit F. Hyg. 177:26. 3.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination ofFoods, 5th Ed., American Public Health Association, Washington, D.C. 4.Standard Methods for the Microbiological Examination of Dairy Products, 1995, 19th Ed, APHA, Washington, D.C. 5.Bacteriological Analytical Manual, 5th Ed, 1978, AOAC, Washington D.C. 6.The European Pharmacopoeia, 2008, Council or Europe, Strasbourg. 7.The British Pharmacopoeia, 2008 vol. II, London. 8.Indian Pharmacopoeia, 2010, Ministry of Health and Family Welfare, Govt., of India, 10.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. 11. Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water and Wastewater,23rd ed., APHA, Washington, D.C. 12..Isenberg, H.D. Clinical Microbiology Procedures Handb0ook. 2nd Edition.1 3.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015)Manual of Clinical Microbiology, 11th Edition. Vol. 1. |