Your enquiry has been submitted
Your enquiry has been submitted
R-2A Agar
Drinking Water#CC293D
Intended Use
Recommended for heterotrophic plate count of water samples using longer incubation periods.
Composition**
| Ingredients | g/L |
|---|---|
| Acicase# | 0.500 |
| Yeast extract | 0.500 |
| Proteose peptone | 0.500 |
| Dextrose (Glucose) | 0.500 |
| Starch soluble | 0.500 |
| Dipotassium hydrogen phosphate | 0.300 |
| Magnesium sulphate | 0.024 |
| Sodium pyruvate | 0.300 |
| Agar | 15.000 |
| Final pH (at 25°C) | 7.2±0.2 |
**Formula adjusted, standardized to suit performance parameters
# Equivalent to Casein Acid Hydrolysate
Directions
Suspend 18.12 grams in 1000 ml purified / distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes or as per validated cycle. DO NOT OVERHEAT. Cool to 45-50°C. Mix well and pour into sterile Petri plates.
Principle And Interpretation
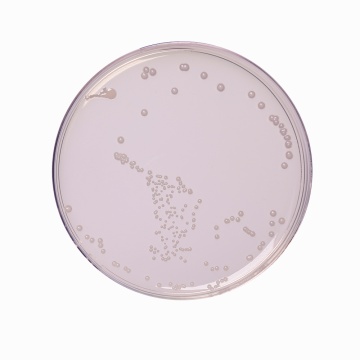
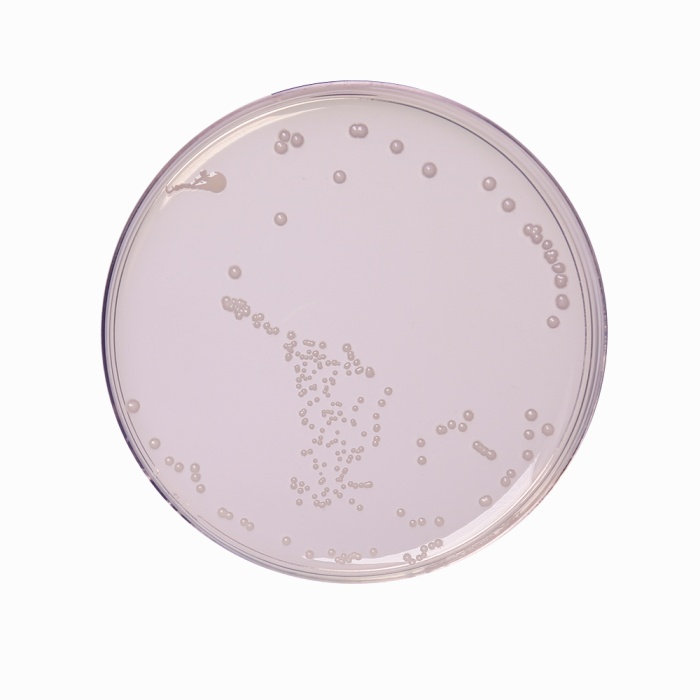
The heterotrophic plate count (HPC), formerly known as the standard plate count is a procedure for estimating the number of live heterotrophic bacteria in water and measuring changes during water treatment, in distribution systems or in swimming pools. R-2A Agar is recommended by APHA (1, 2) for estimating the heterotrophic plate count by the pour plate, spread plate or membrane filter procedure. R-2A Agar is formulated as per Reasoner and Geldreich (3). Stressed or injured organisms during water treatment are unable to grow on high nutrient media, since the faster growing organisms outgrow the former (4). Therefore the use of a low nutrient medium like R-2A Agar incubated for longer incubation periods allows these stressed organisms to grow well.
Many bacteria from natural waters which contain limited nutrients at ambient temperature, grow best on the media with less nutrient levels. They grow better at the temperatures below the routine laboratory incubation temperatures of 35 to 37°C (4).
Acicase, proteose peptone and yeast extract provide nitrogen, carbon compounds, vitamins, amino acids and minerals. Dextrose/ glucose serves as an energy source. Soluble starch aids in the recovery of injured organisms by absorbing toxic metabolic byproducts while sodium pyruvate increases the recovery of stressed cells. Magnesium sulphate is a source of divalent cations and sulphate. Dipotassium hydrogen phosphate is used to balance the pH of the medium. The number of colonies on a plate are reported as CFU (Colony Forming Units) per volume of sample.
Type of specimen
Water samples
Specimen Collection and Handling
For water samples, follow appropriate techniques for sample collection, processing as per guidelines and local standard (1). After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
Read the label before opening the container. Wear protective gloves/protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Longer incubation time other than specified is required for slow growing microorganisms.
- The media is intended for water samples for recovery of stressed or injured organisms.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance
Cream to yellow homogeneous free flowing powder
Gelling
Firm, comparable with 1.5% Agar gel
Colour and Clarity of prepared medium
Light yellow coloured clear to slightly opalescent gel forms in Petri plates
Reaction
Reaction of 1.81% w/v aqueous solution at 25°C. pH : 7.2±0.2
pH
7.00-7.40
Cultural Response
Cultural characteristics observed *by using standard ATCC cultures after an incubation at 30-35°C for 24-72 hours. (*-In case of water samples from fields it is suggested to incubate further for upto 7 days to ascertain the absence of organisms)
| Organism | Inoculum (CFU) | Growth | Recovery |
|---|---|---|---|
| Candida albicans ATCC 10231 (00054*) | 50-100 | good-luxuriant | >=70% |
| Escherichia coli ATCC 25922 (00013*) | 50-100 | good-luxuriant | >=70% |
| Salmonella Enteritidis ATCC 13076 (00030*) | 50-100 | good-luxuriant | >=70% |
| ^Pseudomonas paraeruginosa ATCC 9027 (00026*) | 50-100 | good-luxuriant | >=70% |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | 50-100 | good-luxuriant | >=70% |
| **Bacillus spizizenii ATCC 6633 (00003*) | 50-100 | good-luxuriant | >=70% |
| #Aspergillus brasiliensis ATCC 16404 (00053*) | 50-100 | good-luxuriant | >=70% |
| Enterococcus faecalis ATCC 29212 (00087*) | 50-100 | good-luxuriant | >=70% |
| Salmonella Typhi ATCC 6539 | 50-100 | good-luxuriant | >=70% |
Key: (*) Corresponding WDCM numbers.
**Formerly known as Bacillus subtilis subsp. spizizenii
^ Formerly known as Pseudomonas aeruginosa
# Formerly known as Aspergillus niger
Storage and Shelf Life
Store between 10-30°C in tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product.
Reference
- Lipps WC, Braun-Howland EB, Baxter TE,eds. Standard methods for the Examination of Water and Wastewater, 24th ed. Washington DC:APHA Press; 2023
- Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Reasoner D. J. and Geldreich E. E., 1985, Appl. Environ. Microbiol., 49:
- Collins V. J. and Willoughby J. G., 1962, Arch. Microbiol., 43:294.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.66
| Product Name | R-2A Agar |
|---|---|
| SKU | M962 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1. Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water and Wastewater,Wastewater, 20th Ed., American Public Health Association, Washington, D.C. |