Your enquiry has been submitted
Your enquiry has been submitted
Blood Agar Base No. 2 w/ 1.2% Agar, HiVeg®
Composition
| Ingredients | MV834 Grams/Litre | MV834A Grams/Litre |
|---|---|---|
| HiVeg® peptone No.3 | 15.00 | 15.00 |
| HiVeg® extract No.2 | 2.50 | 2.50 |
| Yeast extract | 5.00 | 5.00 |
| Sodium chloride | 5.00 | 5.00 |
| Agar | 15.00 | 12.00 |
Final pH (at 25°C) 7.4± 0.2
** Formula adjusted, standardized to suit performance parameters
Product Profile
| Vegetable based (Code MV) | Animal based (Code M) | ||
| MV834/MV834A | M834/M834A | ||
| HiVeg® peptone No. 3 | Proteose peptone | ||
| HiVeg® extract No. 2 | Liver extract | ||
Recommended for
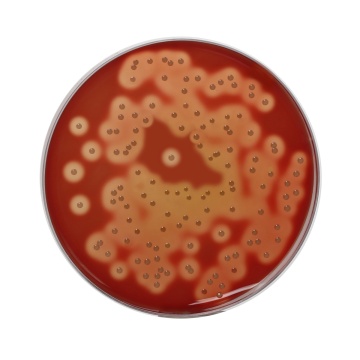
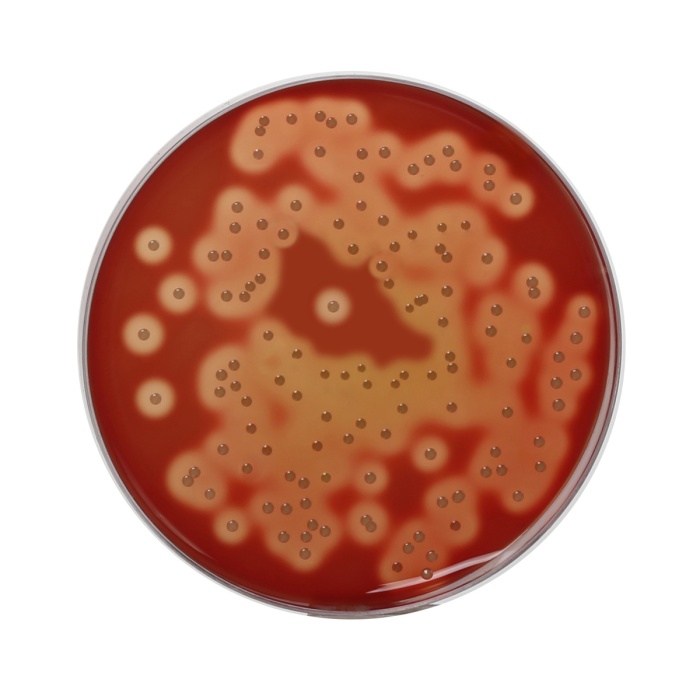
Maximum recovery of fastidious pathogenic microorganisms without interfering with their haemolytic reactions.
Reconstitution
(MV834): 42.5 g/l
(MV834A): 39.5 g/l
Quantity on preparation
| (500g): | (MV834): 11.76 L |
| (100g): | (MV834): 2.35 L |
| (500g): | (MV834A): 12.65 L |
pH (25°C)
7.4± 0.2
Supplement
Defibrinated blood and FD's as desired.
Sterilization
121°C / 15 minutes
Storage
Dry Medium Below 30°C, Prepared Medium 2-8°C.
Directions
Suspend 21.25 grams of MV834 or 19.75 grams of MV834A in 500 ml distilled water. Heat to boiling to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. Cool to 40 - 50°C and aseptically add 7% v/v sterile defibrinated blood.
For Brucella species: Add rehydrated contents of 1 vial of Brucella Selective Supplement (FD005) to 500 ml sterile molten base.
For Campylobacter species: Add rehydrated contents of 1 vial of Campylobacter Supplement - I (FD006) or Campylobacter Supplement - II (FD007) or Campylobacter Supplement - III (FD008) or Campylobacter Growth Supplement (FD009) to 500 ml sterile molten base.
For Streptococci species: Add rehydrated contents of 1 vial of Strepto Supplement (FD031) to 500 ml sterile molten base. Mix well and pour into sterile petri plates.
Principle and Interpretation
These medias are prepared by using vegetable peptones in place of animal peptones, which make the medium free of BSE/TSE risks. These media can be used to prepare a selective medium for Brucella species or Campylobacter species by adding antibiotic supplement selective for respective bacteria (1, 2) like conventional Blood Agar Base. In comparison to other Blood Agar Base, Blood Agar Base No. 2, HiVeg® offers enhanced growth especially for fastidious organisms. Brucella cultures are highly infective and must be handled with care. Incubate preferably in 5-10% carbon dioxide atmosphere. Comparative studies of horse, rabbit and sheep blood were performed by Snavely and Brahier and it was found that sheep blood gave the clearest and most reliable colony and haemolysis characteristics at both 24 and 48 hours (3). These medium can also be used for primary isolation of Haemophilus species, where horse blood is used to enrich the medium. Better results are obtained by spreading half of the horse blood agar plate with 2 drops of 10% saponin (4). Supplementation with blood (5-10%) provides additional growth factors for fastidious microorganisms and is the basis for determining haemolytic reactions. With added HiVeg® extract No.2 and yeast extract the medium shows enhanced growth and haemolytic reactions of fastidious organisms like Streptococci and Pneumococci. HiVeg® peptone No. 3 is the nitrogen source and sodium chloride maintains osmotic equilibrium.
Quality Control
Appearance of Powder
Yellow coloured, may have slightly greenish tinge, homogeneous, free flowing powder.
Gelling
Firm, comparable with 1.5% Agar gel of MV834 or 1.2% Agar gel of MV834A.
Colour and Clarity
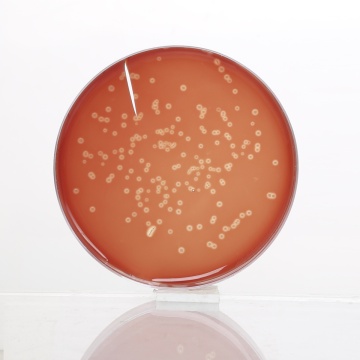
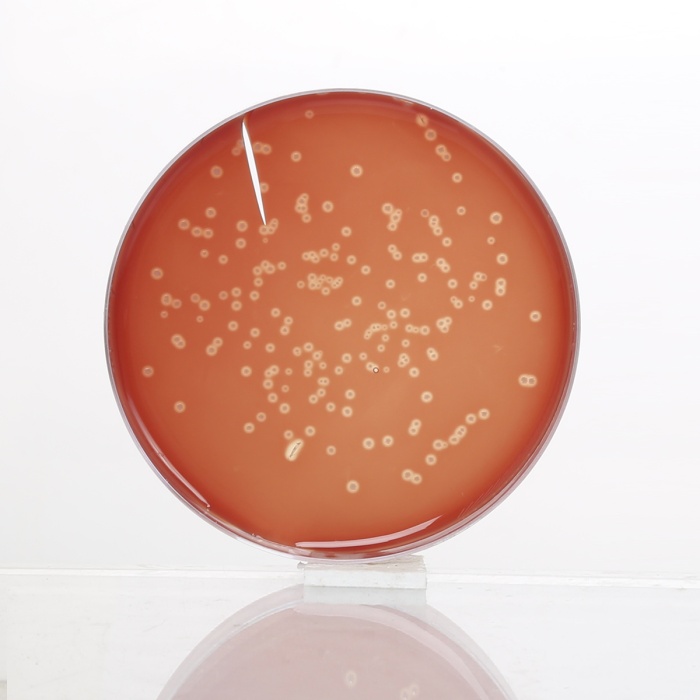
Basal medium yields yellow coloured clear to slightly opalescent gel, with addition of 7% v/v sterile defibrinated blood, cherry red coloured, opaque gel forms in petri plates.
Reaction
Reaction of 4.25% w/v of MV834 or 3.95% w/v of MV834A aqueous solution is pH 7.4± 0.2 at 25°C.
Cultural Response
Cultural characteristics observed after an incubation at 35 - 37°C for 48 hours for MV834 and for 18-48 hours for MV834A.
| Organisms(ATCC) | Inoculum (CFU) | Growth w/blood | Recovery | Haemolysis |
|---|---|---|---|---|
| Neisseria meningitidis (13090) | 102-103 | good to luxuriant | >70% | none |
| Streptococcus pneumoniae (6303) | 102-103 | good to luxuriant | >70% | alpha |
| Streptococcus pyogenes (19615) | 102-103 | good to luxuriant | >70% | beta |
| Staphylococcus aureus (25923) | 102-103 | good to luxuriant | >70% | beta |
| Product Name | Blood Agar Base No. 2 w/ 1.2% Agar, HiVeg® |
|---|---|
| SKU | MV834A |
| Product Type | HiVeg™ |
| Physical Form | Powder |
| Origin | Animal Free (Veg) |
| Packaging type | HDPE |
| References | 1. Norton C. F., 1986, Microbiology, 2nd Edition, Addison-Wesley Publishing Company. |
| Customized Product Available | No |