Your enquiry has been submitted
Your enquiry has been submitted
Violet Red Bile Glucose Agar w/o Lactose
Enterobacteriaceae#CC293D
Intended Use
Recommended for enumeration of Enterobacteriaceae in raw food and clinical samples. The composition and performance criteria are in accordance with ISO 21528-1&2:2017 and ISO 11133:2014/Amd.2: 2020 (Ε).
Composition
ISO Specifications-Violet Red Bile Glucose Agar w/o Lactose
| Ingredients | g/L |
|---|---|
| Enzymatic digest of animal tissues | 7.000 |
| Yeast extract | 3.000 |
| Sodium chloride | 5.000 |
| Bile salts No.3 | 1.500 |
| Glucose | 10.000 |
| Neutral red | 0.030 |
| Crystal violet | 0.002 |
| Agar | 9.000-18.000 |
| Final pH (at 25°C) | 7.4±0.2 |
Violet Red Bile Glucose Agar w/o Lactose
| Ingredients | g/L |
|---|---|
| Peptone $ | 7.000 |
| Yeast extract | 3.000 |
| Sodium chloride | 5.000 |
| Bile salts mixture | 1.500 |
| Glucose (Dextrose) | 10.000 |
| Neutral red | 0.030 |
| Crystal violet | 0.002 |
| Agar | 12.000 |
| Final pH (at 25°C) | 7.4±0.2 |
**Formula adjusted, standardized to suit performance parameters
$ -Equivalent to Enzymatic digest of animal tissues
Directions
Suspend 38.53 gram in 1000 ml purified/distilled water. Heat to boiling to dissolve the medium completely. DO NOT AUTOCLAVE. Cool to 45-50°C. Mix well and pour into sterile Petri plates.
Principle And Interpretation
Violet Red Bile Agar, a modification of MacConkey original formulation (1) is used for the enumeration of coli-aerogenes bacterial group. Violet Red Bile Glucose Agar w/o Lactose, a modification of VRBA (M049), was designed for the enumeration of Enterobacteriaceae (2). It employs the selective inhibitory components crystals violet and bile salts and the indicator system glucose and neutral red. Sought bacteria will dissimilate glucose and produce purple zones around the colonies (3). ISO committee has also recommended this medium (4,5). Selectivity of VRBGA can be increased by incubation under anaerobic conditions and/or at elevated temperature, i.e. equal to or above 42°C (6-8).
Peptone and yeast extract serve as sources of carbon, nitrogen, vitamins and other essential growth nutrients. Glucose is the fermentable carbohydrate, utilization of which leads to the production of acids. Neutral red indicator detects the acidity so formed. Crystal violet and bile salts mixture help to inhibit the accompanying gram-positive and unrelated flora. Sodium chloride maintains the osmotic equilibrium. Further biochemical tests are necessary for positive identification (9).
Type of specimen
Clinical samples - faeces; Food and dairy samples; Water samples
Specimen Collection and Handling
For clinical samples follow appropriate techniques for handling specimens as per established guidelines (10,11).
For food and dairy samples, follow appropriate techniques for sample collection and processing as per guidelines (4,5,12-14).
For water samples, follow appropriate techniques for sample collection, processing as per guidelines & local standards (15).
After use, contaminated materials must be sterilized by autoclaving before discarding.
Warning and Precautions
In Vitro diagnostic use. For professional use only. Read the label before opening the container. Wear protective gloves/ protective clothing/eye protection/face protection. Follow good microbiological lab practices while handling specimens and culture. Standard precautions as per established guidelines should be followed while handling clinical specimens. Safety guidelines may be referred in individual safety data sheets.
Limitations
- Individual organisms differ in their growth requirement and may show variable growth patterns on the medium.
- Each lot of the medium has been tested for the organisms specified on the COA. It is recommended to users to validate the medium for any specific microorganism other than mentioned in the COA based on the user's unique requirement.
- Over incubation may result in reverting of reaction.
- Further biochemical tests must be carried out on colonies of pure culture for confirmation.
Performance and Evaluation
Performance of the medium is expected when used as per the direction on the label within the expiry period when stored at recommended temperature.
Quality Control
Appearance
Light yellow to pinkish beige homogeneous free flowing powder
Gelling
Firm,comparable with 1.2% Agar gel.
Colour and Clarity of prepared medium
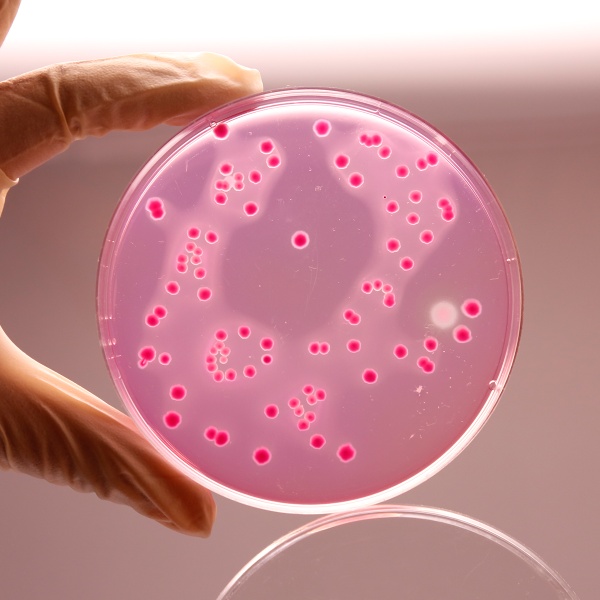
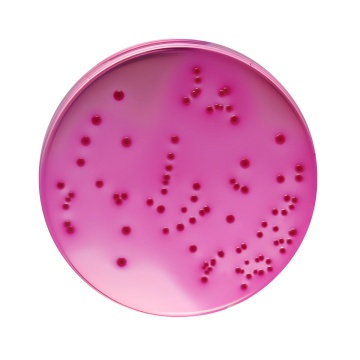
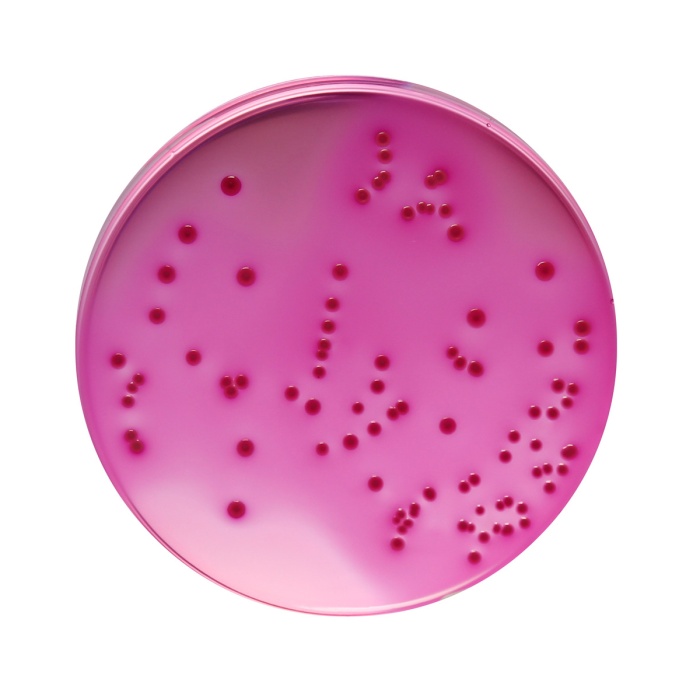
Reddish purple coloured clear to slightly opalescent gel forms in Petri plates.
Reaction
Reaction of 3.85% w/v of aqueous solution at 25°C. pH: 7.4±0.2
pH
7.20-7.60
Cultural Response
Productivity: Cultural characteristics was observed after an incubation at 35±1°C for 24±2 hours. Recovery rate is considered as 100% for bacteria growth on Soyabean Casein Digest Agar.
Selectivity: Cultural characteristics was observed after an incubation at 35±1°C for 24±2 hours.
| Organism | Inoculum (CFU) | Growth | Recovery | Colour of colony |
|---|---|---|---|---|
| Productivity | ||||
| Escherichia coli ATCC 25922 (00013*) | 50-100 | luxuriant | >=50% | pink to red colonies with or without precipitation zone |
| Escherichia coli ATCC 8739 (00012*) | 50-100 | luxuriant | >=50% | pink to red colonies with or without precipitation zone |
| Salmonella Enteritidis ATCC 13076 (00030*) | 50-100 | luxuriant | >=50% | pink to red colonies with or without precipitation zone |
| Salmonella Typhimurium ATCC 14028 (00031*) | 50-100 | luxuriant | >=50% | pink to red colonies with or without precipitation zone |
| Selectivity | ||||
| Enterococcus faecalis ATCC 29212 (00087*) | >=104 | inhibited | ||
| Enterococcus faecalis ATCC 19433 (00009*) | >=104 | inhibited | ||
Key: (*) Corresponding WDCM numbers.
Storage and Shelf Life
Store between 10-30°C in a tightly closed container and the prepared medium at 20-30°C. Use before expiry date on the label. On opening, product should be properly stored dry, after tightly capping the bottle in order to prevent lump formation due to the hygroscopic nature of the product. Improper storage of the product may lead to lump formation. Store in dry ventilated area protected from extremes of temperature and sources of ignition. Seal the container tightly after use. Product performance is best if used within stated expiry period.
Disposal
User must ensure safe disposal by autoclaving and/or incineration of used or unusable preparations of this product. Follow established laboratory procedures in disposing of infectious materials and material that comes into contact with clinical sample must be decontaminated and disposed of in accordance with current laboratory techniques (10,11).
Reference
- MacConkey A., 1905, J. Hyg., 5, 333-379.
- Mossel D. A. A., Eclderink I., Koopmans M. and Van Rossem F., 1978, Lab. practice, 27 No. 12: 1049.
- Corry J. E. L., Curtis G. D. W. and Baird R. M., (Ed.), 1995, Culture Media for Food Microbiology, Vol. 34, Progress in Industrial Microbiology, Elsevier, Amsterdam.
- Microbiology of the food chain Horizontal method for the detection and enumeration of Enterobacteriaceae Part 2: Colony-count technique ISO 21528-2:2017.
- Microbiology of food, animal feeding stuffs and water- Preparation, production, storage and performance testing of culture media, EN ISO 11133:2014 (E) /Amd.: 2020
- Mossel D. A. A. and Vega C. L., 1973, Hlth. Lab. Sci., 11:303
- Mossel D. A. A., Eclderink I., Koopmans M. and Van Rossem F., 1979, Food Protect., 42: 470
- Mossel D. A. A. et al, 1986, J. Appl. Bacteriol., 60:289.
- MacFaddin J. F., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. 1, Williams and Wilkins, Baltimore.
- Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition.Clinical Microbiology, 11th Edition. Vol.
- Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual of Clinical Microbiology, 11th Edition. Vol. 1.
- American Public Health Association, Standard Methods for the Examination of Dairy Products, 1978, 14th Ed., Washington D.C.
- Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods, 5th Ed., American Public Health Association, Washington, D.C.
- Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed., APHA Inc., Washington, D.C.
- Lipps WC, Braun-Howland EB, Baxter TE,eds. Standard methods for the Examination of Water and Wastewater, 24th ed. Washington DC:APHA Press; 2023.
| Product Name | Violet Red Bile Glucose Agar w/o Lactose |
|---|---|
| SKU | M581 |
| Product Type | Regular |
| Physical Form | Powder |
| Origin | Animal |
| Packaging type | HDPE |
| References | 1.American Public Health Association, Standard Methods for the Examination of Dairy Products, 1978, 14th Ed., WashingtonD.C. 2.Baird R.B., Eaton A.D., and Rice E.W., (Eds.), 2015, Standard Methods for the Examination of Water and Wastewater, 23rded., APHA, Washington, D.C. 3.Corry J. E. L., Curtis G. D. W. and Baird R. M., (Ed.), 1995, Culture Media for Food Microbiology, Vol. 34, Progress inIndustrial Microbiology, Elsevier, Amsterdam. 4.International Organization for Standardization (ISO), 1993, Draft ISO/DIS 7402. 5.Isenberg, H.D. Clinical Microbiology Procedures Handbook. 2nd Edition. 6.Jorgensen,J.H., Pfaller , M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S and Warnock., D.W. (2015) Manual ofClinical Microbiology, 11th Edition. Vol. 1. 7.MacConkey A., 1905, J. Hyg., 5, 333-379. 8.MacFaddin J. F., 1985, Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria, Vol. 1, Williamsand Wilkins, Baltimore9.Mossel D. A. A., Eclderink I., Koopmans M. and Van Rossem F., 1978, Lab. practice, 27 No. 12: 1049.10.Mossel D. A. A. and Vega C. L., 1973, Hlth. Lab. Sci., 11:303 13.Salfinger Y., and Tortorello M.L. Fifth (Ed.), 2015, Compendium of Methods for the Microbiological Examination of Foods,5th Ed., American Public Health Association, Washington, D.C. 14.Wehr H. M. and Frank J. H., 2004, Standard Methods for the Microbiological Examination of Dairy Products, 17th Ed.,APHA Inc., Washington, D.C. |