Compliances of IVD to IVDR and Indian MDR standards
Compliances of IVD to IVDR and Indian MDR standards
HiMedia's constant commitment to ensure high standards of safety, quality and performance of in-vitro diagnostic products for use in the health care sector contributes directly and indirectly to the well being of patients across the country and world-wide.
Since 2004 HiMedia Laboratories has adapted International agreed standards, ISO 13485 Quality Management Systems; that fulfills statutory and regulatory requirements that are specific to medical devices and in-vitro diagnostic devices.
Today WHO GMP certifications, ISO 9001:2015 and ISO 13485:2016 Quality Management Systems of HiMedia allows complete traceability of In-vitro diagnostic products throughout its life cycle from inception, development, product realization, and decommissioning stages.
INTRODUCTION
What are In-Vitro Diagnostic (IVD) Medical Devices?
In-Vitro Diagnostic Medical Devices (IVD products) are devices and systems used to diagnose, treat, or prevent health conditions. They are meant to be used in the collection and examination of biological samples of human origin such as urine, faeces, blood, saliva, body fluids, tissue, secretions, swab samples, etc. Samples may be taken from inside the nose or the back of the throat, or from a vein or fingerstick.
It's important to note that IVD products are noninvasive. However, they are still considered under category of medical devices and are therefore subject to the same controls. They are also referred to as IVD Kits /Reagents or in-vitro diagnostic substance.
Are In-Vitro Diagnostic products regulated in India?
Yes
All In -Vitro Diagnostic kits/reagents are regulated in India under the provisions of the Medical Devices Rules, 2017 and Medical Devices Amendment (2020).
Manufacturing License is granted by
Central Drugs Standard Control Organisation (CDSCO) under Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India.
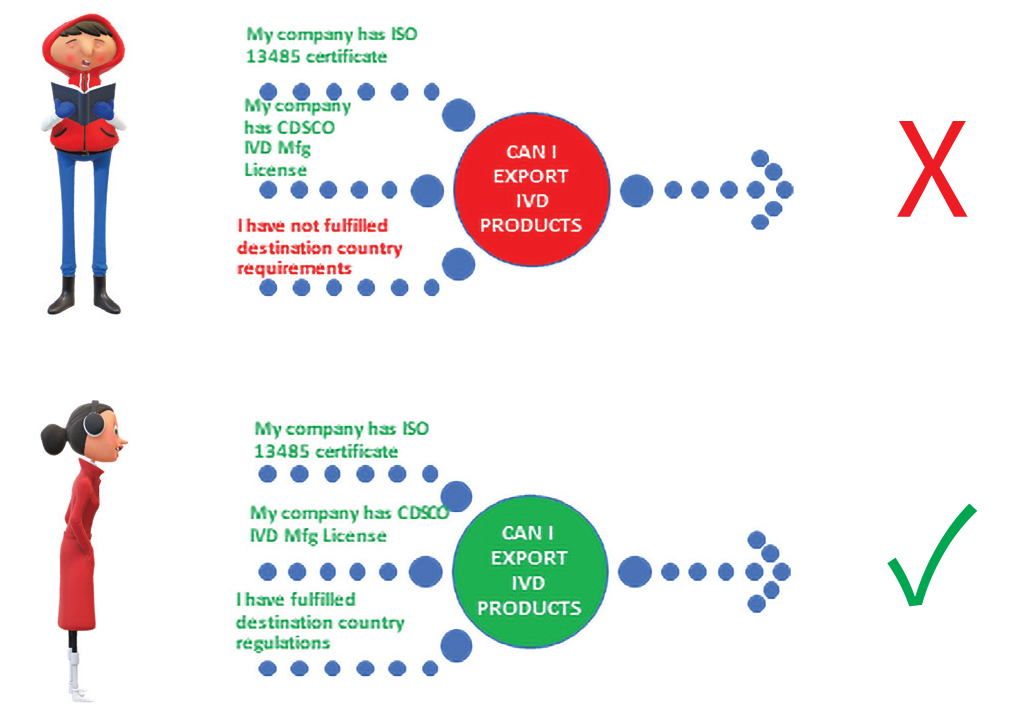
If the manufacturer has fulfilled Indian Regulation does he need to fulfill other country rules?
Yes
Manufacturers of In-Vitro Diagnostics must meet the regulatory requirements of the destination country which they wish to penetrate and supply the products.
Are the regulations same in India and other countries?
No The purpose may be same but they are regulated by their National authorities under provisions of respective Acts/ Regulations.
An intermediate legal entity; Authorized representative acts locally on behalf of non-native supplier of IVD products.
Global Recognition of HiMedia as one of the leading manufacturers & suppliers of IVD products
• As products are registered in different countries fulfilling their medical device regulations
• As Himedia exports products in more than 150 countries
Why are IVD products regulated?
If the testing results obtained using IVD products are found to be inaccurate it can have a negative impact on patient's health and pose certain level of risk. Inorder to control any sort of such circumstances leading to an adverse event, IVD products are regulated.
Based on the impact it poses to public and the person handling the device, risks have been classified universally by the World Health Organization (WHO) as Low-risk, Moderate-risk and High-risk.
What is Risk-Based Classification of IVD products?
IVDs are classified according to the health risk (either to the public or an individual) that may arise from an incorrect result.
| Classification of IVD products | Risk Level |
|---|---|
| Class A | Public health and personal risk low |
| Class B | Public health risk Low; Personal risk moderate to low |
| Class C | Public health risk moderate - low; Personal risk low |
| Class D | High Public health and Personal risk |
India In-Vitro Diagnostics Companies List
- HiMedia Laboratories Private Limited
- Abbott Laboratories
- Arkray Inc.
- Becton, Dickinson and Company
- BioMérieux SA
- Bio-Rad Laboratories Inc.
India In-Vitro Diagnostics Companies List
- Danaher Corporation
- F. Hoffmann-La Roche AG
- Merck India
- Transasia Bio-Medicals Ltd
- Sysmex Corporation
- Thermo Fisher Scientific
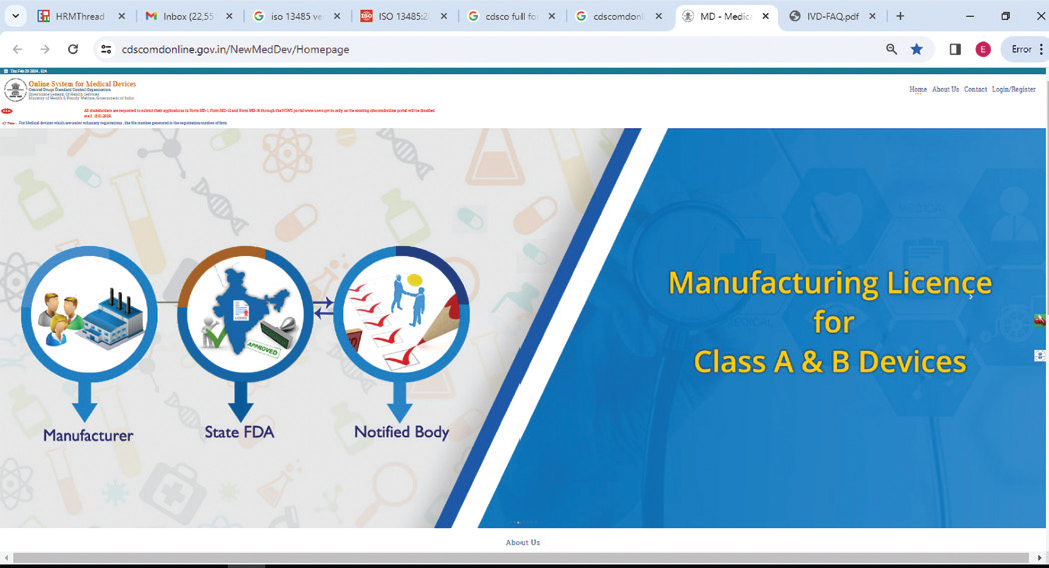
Registration under Indian MDR
In India, Applications are submitted online in the SUGAM portal for the grant of license and registration of Medical devices and In-vitro diagnostic product by Central Drugs Standard Control Organization (CDSCO). Refer https:// cdscomdonline.gov.in/
IVD are classified as Class A, B, C, or D as per IMDR 2017 Risk- Based Classification Approach.
| CLA (Central Licensing Authority ) Conducts audit for Class C & D applications and is authorized to grant licenses of these high-risk group products | SLA (State Licensing Authority ) Conducts audit for Class A & B applications and is authorized to grant licenses of these low & moderate risk group products |
Only SLA (State Licensing Authority) appoints Notified Bodies to carry out this activity. For Class A audit, FDA authorities or Notified Body on behalf of SLA may inspect plant for its approval.
Registration under EU IVDR
For a Non-European Manufacturer, The European Authorized Representative chosen serves as a liaison between the Non- European Manufacturer and the national Competent Authorities (Ministries of Health) who shall assist with medical device and IVD registrations, as required. Refer https://euivdr.com/

IVD are classified as Class A Non-sterile & Sterile, B, C, or D as per IVDR 2017/746 Risk-Based Classification Approach.
| IVDs that fall under Nonsterile Class A are selfcertified and need to involve Authorized representative for registrations in Member State Competent authority | IVDR in EU has made it mandatory for IVDs Manufacturers to get approval from Notified Body for Class A sterile, B, C or D class. |
There will be no involvement of an Authorized representative for Class A sterile, B, C, or D risk class products. Devices are registered in EU and CE certificate is granted when the product has proven record of clinical safety and performance of the device.
Recent CDSCO update for Class C or D registrations under IMDR 2017 (Indian Medical Device Rule 2017)
Importers and manufacturers who have already filed the application to Central Licensing Authority on or before 30th September 2023, can continue to import or manufacture the mentioned devices for up to six months from the date of this order or until the Central Licensing Authority makes a decision on the applications, whichever comes first.
The latest update for registrations under IVDR (In-Vitro Diagnostic Regulation 2017/746)
Extension period has been granted for IVD products other than Nonsterile Class A.
26 May 2025 for high-risk IVDs Class D
26 May 2026 for high-risk IVVDs Class C
26 May 2027 for low-risk IVDs Class B & Class A Sterile
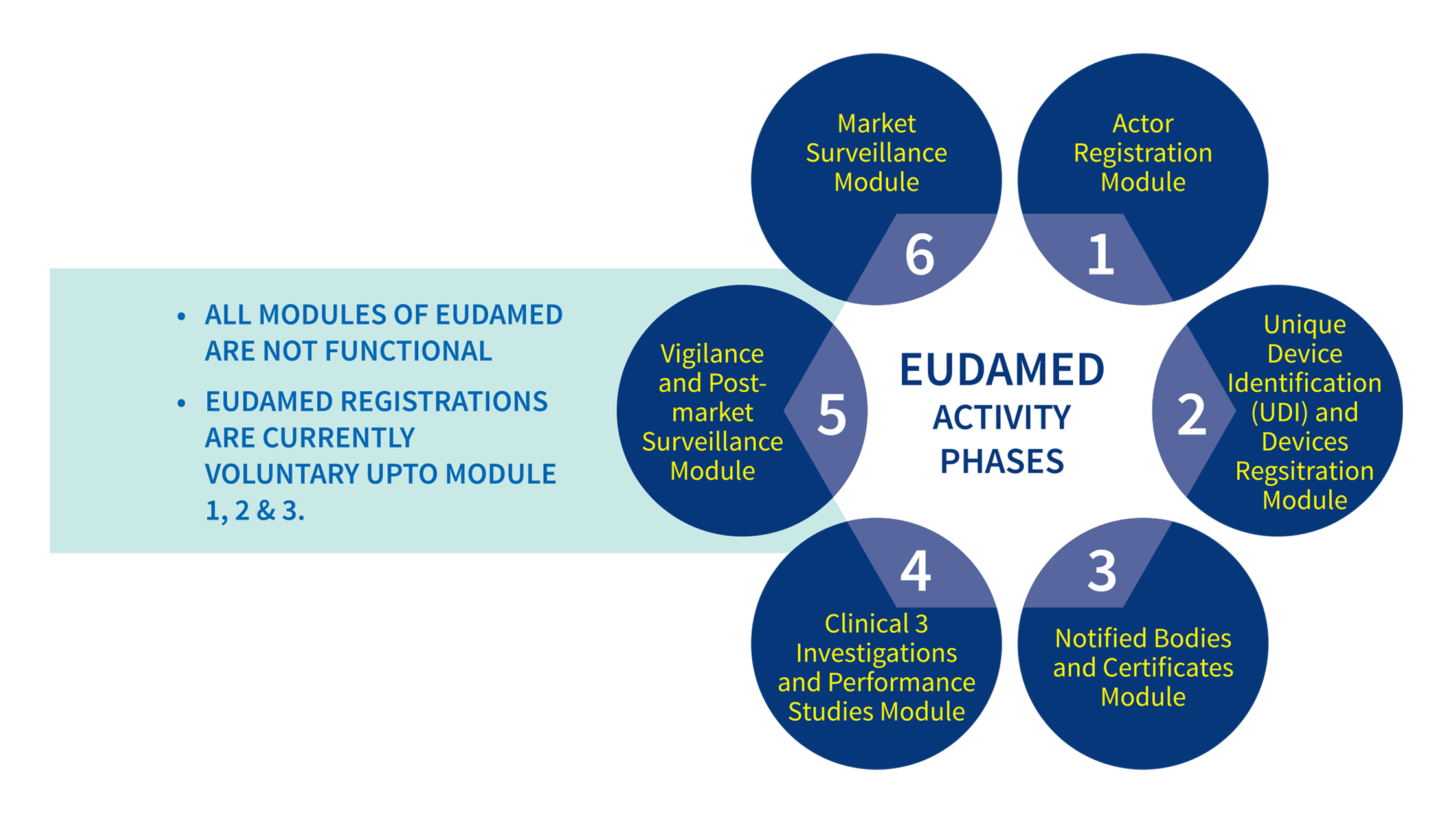
Gradual Roll out of EUDAMED, the new electronic data base
Way forward, comparison and compliances to IMDR and EU IVDR
At present there is no central safety database of invitro diagnostic products in India which is accessible to the public. On other hand, the EU has the European Databank on Medical Devices (EUDAMED).
EUDAMED is the IT system developed by the European Commission to implement provisions of Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnosis medical devices.
EUDAMED is a new European system for medical devices that aims to put all aspects of registering, monitoring, and managing information about products among different industry actors.
EUDAMED is composed of six modules. They are Actor registration, Unique device identification (UDI) and device registration, Notified bodies and certificates, Clinical investigations and performance studies, Vigilance and Market surveillance.
Though there is relaxation in timelines for product registrations, IVD manufacturers & suppliers of IVD products to EU are required to fulfill new rules.
EU Commission 2010/227 has enforced an electronic database EUDAMED wherein it is expected to :
• Provide information about their products via the completed EUDAMED modules.
• Mandatory registration in EUDAMED is expected to take effect as of late 2025.
Once fully operational, EUDAMED will function as a registration system, a collaborative system, a notification system, and a dissemination system (to the public) and it will be interoperable with other existing and upcoming systems managed by the European Commission, EMA, and Member States
References
- https://cdsco.gov.in/opencms/opencms/en/Medical- Device-Diagnostics/InVitro-Diagnostics
- https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/ Pdf-documents/IVD/FAQs/AnnexureIIIVD.pdf
- https://eur-lex.europa.eu/legal-content/EN/TXT/ PDF/?uri=CELEX:32017R0746
- https://eur-lex.europa.eu/LexUriServ/LexUriServ. do?uri=CONSLEG:1998L0079:20090807:en:PDF
- https://health.ec.europa.eu/system/files/2023-10/md_ eudamed_roadmap_en.pdf
- https://health.ec.europa.eu/medical-devices-eudamed/ overview_en
Glossary
| CE | Certificate of Conformite Europeenne (French word for European Conformity) |
| Compliance | Adherence to applicable Rules & Laws |
| EU | European Union |
| EUDAMED | European DataBank on Medical Devices |
| IVD | In-Vitro Diagnostic Devices |
| IVDD | In-Vitro Diagnostic Device Directive 98/79 EC |
| IVDR | In-Vitro Diagnostic Regulation 2017/746 |
| IMDR | Indian Medical Device Rule, 2017 |
| MDR | Medical Device Rule |
| QMS | Quality Management Systems |